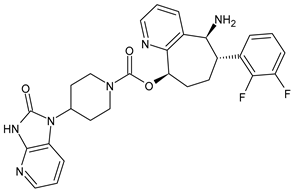
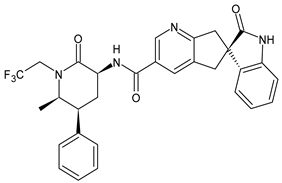
Migraine must not be confused with a simple headache; it is a serious and disabling disease that causes considerable limitations in the daily life of afflicted people, including social, work, and emotional effects. Therefore, it causes a daily state of suffering and discomfort. It is important to point out that this pathology not only has a decisive impact on the quality of life of those who suffer from it but also on their families and, more generally, on society as a whole. The clinical picture of migraine is complex, with debilitating unilateral or bilateral head pain, and is often associated with characteristic symptoms such as nausea, vomiting, photophobia, and phonophobia. Hormonal, environmental, psychological, dietary, or other factors can trigger it.
- migraine
- CGRP
- gepants
- headache
- ketogenic diet
1. Introduction
2. Classification of Headaches
3. Calcitonin Gene-Related Peptide (CGRP) Receptor
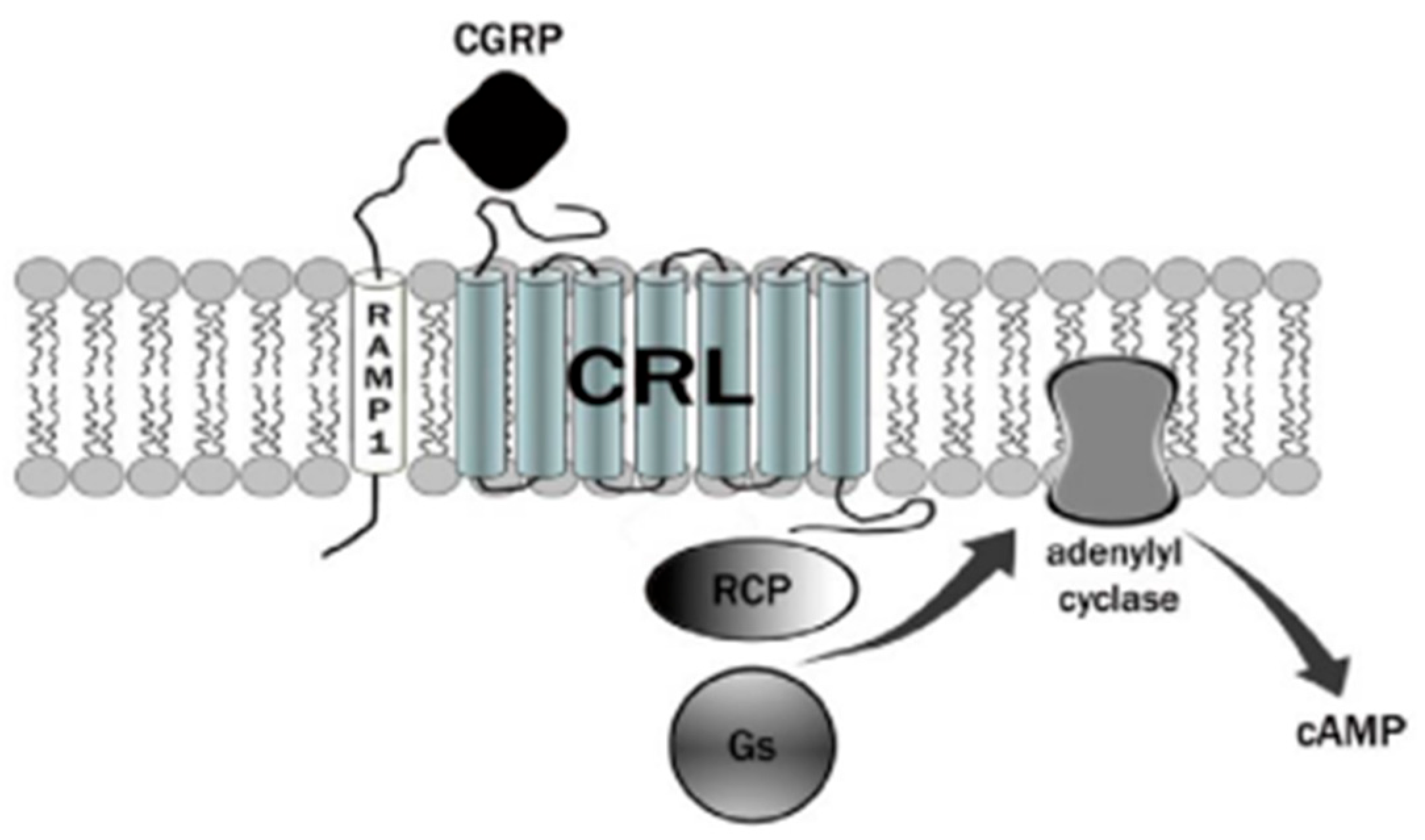
4. Therapies Based on the Different Forms of Migraine
|
Structure |
Name |
Class |
|---|---|---|
|
|
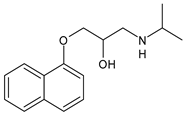
Propranolol |
β-blocker |
|
|
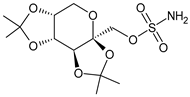
Topiramate |
β-blocker |
|
|
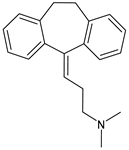
Amitriptyline |
Antidepressant |
|
|
Ibuprofen |
FANS |
|
|
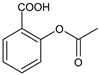
Acetylsalicylic acid |
FANS |
|
|
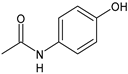
Acetaminophen |
FANS |
|
|
Indomethacin |
FANS |
|
|
Metoclopramide |
Anti-emetic |
|
|
Capsaicin |
Analgesic |
|
|
Olcegepant |
CGRP inhibitor |
|
|
Telcagepant |
CGRP inhibitor |
|
|
Atogepant |
CGRP inhibitor |
|
|
Rimegepant |
CGRP inhibitor |
|
|
Ubrogepant |
CGRP inhibitor |
5. Ketogenic Diet
This entry is adapted from the peer-reviewed paper 10.3390/medicina60010163
References
- Steiner, T.J.; Stovner, L.J. Global epidemiology of migraine and its implications for public health and health policy. Nat. Rev. Neurol. 2023, 19, 109–117.
- Tiwari, R.; Tiwari, G.; Mishra, S.; Ramachandran, V. Preventive and therapeutic aspects of migraine for patient care: An insight. Curr. Mol. Pharmacol. 2023, 16, 147–160.
- Mangrum, R.; Gerstein, M.T.; Hall, C.J., III; Buse, D.C.; Houts, C.R.; McGinley, J.S.; McCarrier, K.P.; Lipton, R.B.; Wirth, R.J. Priority acute and preventive migraine treatment benefits: Results of the Migraine Clinical Outcome Assessment System (MiCOAS) qualitative study of people living with migraine. Headache, 2023; in press.
- Silvestro, M.; Iannone, L.F.; Orologio, I.; Tessitore, A.; Tedeschi, G.; Geppetti, P.; Russo, A. Migraine treatment: Towards new pharmacological targets. Int. J. Mol. Sci. 2023, 24, 12268.
- Ursitti, F.; Valeriani, M. Migraine in childhood: Gender differences. Eur. J. Paediatr. Neurol. 2023, 42, 122–125.
- Marti-Marca, A.; Vilà-Balló, A.; Cerda-Company, X.; Ikumi, N.; Torres-Ferrus, M.; Caronna, E.; Gallardo, V.J.; Alpuente, A.; Torralba Cuello, M.; Soto-Faraco, S.; et al. Exploring sensory sensitivity, cortical excitability, and habituation in episodic migraine, as a function of age and disease severity, using the pattern-reversal task. J. Headache Pain 2023, 24, 104.
- Freddi, T.A.L.; Ottaiano, A.C.; Lucio, L.L.; Corrêa, D.G.; Hygino da Cruz, L.C., Jr. The trigeminal nerve: Anatomy and pathology. Semin. Ultrasound CT MR 2022, 43, 403–413.
- Bigal, M.E.; Lipton, R.B. Modifiable risk factors for migraine progression (or for chronic daily headaches)—Clinical lessons. Headache 2006, 46 (Suppl. S3), S144–S146.
- Pezzella, P. The ICD-11 is now officially in effect. World Psych. 2022, 21, 331.
- Puledda, F.; Wang, S.J.; Diener, H.C.; Schytz, H.W. A history of International Headache Society grants and their impact on headache careers. Cephalalgia 2022, 42, 1288–1293.
- Silva-Néto, R.; Holle-Lee, D. Headaches Classification. In Hypnic Headache; Springer: Cham, Switzerland, 2023.
- Olesen, J. Classification of migraine and tension-type headache. Cephalalgia 2023, 43, 03331024221139238.
- Greenbaum, T.; Emodi-Perlman, A. Headache and orofacial pain: A traffic-light prognosis-based management approach for the musculoskeletal practice. Front. Neurol. 2023, 14, 1146427.
- Fan, X.; Fu, G.; Wang, L.; Shen, W.; Zhang, Y. A bibliometric analysis and visualization of tension-type headache. Front. Neurol. 2022, 13, 980096.
- May, A.; Evers, S.; Goadsby, P.J.; Leone, M.; Manzoni, G.C.; Pascual, J.; Carvalho, V.; Romoli, M.; Aleksovska, K.; Pozo-Rosich, P. European Academy of Neurology guidelines on the treatment of cluster headache. Eur. J. Neurol. 2023; in press.
- Martelletti, P. Non-migraine primary headaches in medicine: A machine-generated overview of current research. In A Machine-Generated Overview of Current Research; Springer: Berlin/Heidelberg, Germany, 2023; ISBN 978-3031208935.
- Russo, A.F.; Hay, D.L. CGRP physiology, pharmacology, and therapeutic targets: Migraine and beyond. Physiol. Rev. 2023, 103, 1565–1644.
- Kamm, K. CGRP and migraine: What have we learned from measuring CGRP in migraine patients so far? Front. Neurol. 2022, 13, 930383.
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—Successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350.
- Pellesi, L.; Guerzoni, S.; Pini, L.A. Spotlight on anti-CGRP monoclonal antibodies in migraine: The clinical evidence to date. Clin. Pharmacol. Drug Dev. 2017, 6, 534–547.
- Hoare, S.R. Mechanisms of peptide and nonpeptide ligand binding to class B G-protein-coupled receptors. Drug Discov. Today 2005, 10, 417–427.
- Scuteri, D.; Tonin, P.; Nicotera, P.; Bagetta, G.; Corasaniti, M.T. Real world considerations for newly approved CGRP receptor antagonists in migraine care. Exp. Rev. Neurother. 2022, 22, 221–230.
- Yao, G.; Man, Y.-H.; Li, A.-R.; Guo, Y.; Dai, Y.; Wang, P.; Zhou, Y.-F. NO up-regulates migraine-related CGRP via activation of an Akt/GSK-3β/NF-κB signaling cascade in trigeminal ganglion neurons. Aging 2020, 12, 6370–6384.
- Karsan, N.; Gosalia, H.; Goadsby, P.J. Molecular mechanisms of migraine: Nitric oxide synthase and neuropeptides. Int. J. Mol. Sci. 2023, 24, 11993.
- Gaete, P.S.; Lillo, M.A.; Puebla, M.; Poblete, I.; Figueroa, X.F. CGRP signalling inhibits NO production through pannexin-1 channel activation in endothelial cells. Sci. Rep. 2019, 9, 7932.
- Blumenfeld, A.M.; Kaur, G.; Mahajan, A.; Shukla, H.; Sommer, K.; Tung, A.; Knievel, K.L. Effectiveness and safety of chronic migraine preventive treatments: A systematic literature review. Pain Ther. 2022, 12, 251–274.
- Lampl, C.; Versijpt, J.; Amin, F.M.; Deligianni, C.I.; Gil-Gouveia, R.; Jassal, T.; MaassenVanDenBrink, A.; Ornello, R.; Paungarttner, J.; Sanchez-Del-Rio, M.; et al. European Headache Federation (EHF) Critical Re-Appraisal and Meta-Analysis of Oral Drugs in Migraine Prevention-Part 1: Amitriptyline. J. Headache Pain 2023, 24, 39.
- McLean, G.; Mercer, S.W. Chronic migraine, comorbidity, and socioeconomic deprivation: Cross-sectional analysis of a large nationally representative primary care database. J. Comorb. 2017, 7, 89–95.
- Krymchantowski, A.V.; Silva-Néto, R.P.; Jevoux, C.; Krymchantowski, A.G. Indomethacin for refractory COVID or post-COVID headache: A retrospective study. Acta Neurol. Belg. 2022, 122, 465–469.
- Ceramella, J.; Iacopetta, D.; Sinicropi, M.S.; Andreu, I.; Mariconda, A.; Saturnino, C.; Giuzio, F.; Longo, P.; Aquaro, S.; Catalano, A. Drugs for COVID-19: An update. Molecules 2022, 27, 8562.
- Rissardo, J.P.; Caprara, A.L.F. Gepants for acute and preventive migraine treatment: A narrative review. Brain Sci. 2022, 12, 1612.
- Sacco, S.; Amin, F.M.; Ashina, M.; Bendsten, L.; Deligianni, C.I.; Gil-Gouveia, R.; Katsarava, Z.; MassenVanDenBrink, A.; Martelletti, P.; Mitsikostas, D.D.; et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention—2022 update. J. Headache Pain 2022, 23, 67.
- Castrillo, A.; Mendoza, A.; Caballero, L.; Cerdán, D.; Rodríguez, M.F.; Guerrero, P.; Tabernero, C.; Ferrero, M.; Benito, I.; Marín, I.; et al. Effectiveness of anti-CGRP monoclonal antibodies in the preventive treatment of migraine: A prospective study of 63 patients. Med. Clínica 2023, 160, 341–346.
- Al-Karagholi, M.A.M.; Kalatharan, V.; Fagerberg, P.S.; Amin, F.M. The vascular role of CGRP: A systematic review of human studies. Front. Neurol. 2023, 14, 1204734.
- Masood, W.; Annamaraju, P.; Uppaluri, K.R. Ketogenic Diet. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Han, X.; Yu, S. Non-Pharmacological Treatment for Chronic Migraine. Curr. Pain Headache Rep. 2023, 27, 663–672.
- Hokenek, N.M.; Erdogan, M.O.; Hokenek, U.D.; Algin, A.; Tekyol, D.; Seyhan, A.U. Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: A randomize controlled trial. Am. J. Emerg. Med. 2020, 39, 80–85.
- De Luca, A.; Talon, S.; de Bellis, M.; Desaphy, J.-F.; Franchini, C.; Lentini, G.; Catalano, A.; Corbo, F.; Tortorella, V.; Conte-Camerino, D. Inhibition of skeletal muscle sodium currents by mexiletine analogues: Specific hydrophobic interactions rather than lipophilia per se account for drug therapeutic profile. Naunyn Schmiedeberg’s Arch. Pharmacol. 2003, 367, 318–327.
- Valente, M.; Garbo, R.; Filippi, F.; Antonutti, A.; Ceccarini, V.; Tereshko, Y.; Di Lorenzo, C.; Gigli, G.L. Migraine prevention through ketogenic diet: More than body mass composition changes. J. Clin. Med. 2022, 11, 4946.
- Lovati, C.; D’Alessandro, C.M.; Della Ventura, S.; Muzio, F.; Pantoni, L. Ketogenic diet in refractory migraine: Possible efficacy and role of ketone bodies—A pilot experience. Neurol. Sci. 2022, 43, 6479–6485.
- Moskatel, L.S.; Zhang, N. Migraine and diet: Updates in understanding. Curr. Neurol. Neurosci. Rep. 2022, 22, 327–334.
- Roehl, K.; Falco-Walter, J.; Ouyang, B.; Balabanov, A. Modified ketogenic diets in adults with refractory epilepsy: Efficacious improvements in seizure frequency, seizure severity, and quality of life. Epilepsy Behav. 2019, 93, 113–118.
- Barbanti, P.; Fofi, L.; Aurilia, C.; Egeo, G.; Caprio, M. Ketogenic diet in migraine: Rationale, findings and perspectives. Neurol. Sci. 2017, 38 (Suppl. S1), 111–115.
- Neri, L.D.C.L.; Ferraris, C.; Catalano, G.; Guglielmetti, M.; Pasca, L.; Pezzotti, E.; Carpani, A.; Tagliabue, A. Ketosis and migraine: A systematic review of the literature and meta-analysis. Front. Nutrit. 2023, 10, 1204700.
- Murakami, M.; Tognini, P. Molecular mechanisms underlying the bioactive properties of a ketogenic diet. Nutrients 2022, 14, 782.
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52.
- Christensen, R.H.; Gollion, C.; Amin, F.M.; Moskowitz, M.A.; Hadjikhani, N.; Ashina, M. Imaging the inflammatory phenotype in migraine. J. Headache Pain 2022, 23, 60.
- Reducha, P.V.; Edvinsson, L.; Haanes, K.A. Could experimental inflammation provide better understanding of migraines? Cells 2022, 11, 2444.
- Lim, J.-M.; Letchumanan, V.; Tan, L.T.-H.; Hong, K.-W.; Wong, S.-H.; Ab Mutalib, N.-S.; Lee, L.-H.; Law, J.W.-F. Ketogenic diet: A dietary intervention via gut microbiome modulation for the treatment of neurological and nutritional disorders (a narrative review). Nutrients 2022, 14, 3566.
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 309–319.
- Caminha, M.C.; Moreira, A.B.; Matheus, F.C.; Rieger, D.K.; Moreira, J.D.; Dalmarco, E.M.; Demarchi, I.G.; Lin, K. Efficacy and tolerability of the ketogenic diet and its variations for preventing migraine in adolescents and adults: A systematic review. Nutr. Rev. 2021, 80, 1634–1647.
- Bu, X.X.; Zhu, L.H.; Wang, Z.M.; Lu, C.; Chen, H.; Yu, D. Association of obesity with headache among US children and adolescents: Evidence from NHANES 1999–2004. Front. Endocrinol. 2023, 13, 1072419.
- Jahromi, S.R.; Martami, F.; Morad Soltani, K.; Togha, M. Migraine and obesity: What is the real direction of their association? Exp. Rev. Neurother. 2023, 23, 75–84.
- Katalinic, D.; Vcev, A.; Smolic, M.; Aleric, I. Serotonin receptor agonists in the treatment of migraine: A meta-analysis considering possible connection with paresthesia. Ann. Ind. Acad. Neurol. 2022, 25, 332.
- Gollion, C.; De Icco, R.; Dodick, D.W.; Ashina, H. The premonitory phase of migraine is due to hypothalamic dysfunction: Revisiting the evidence. J. Headache Pain 2022, 23, 158.
- Bic, Z.; Blix, G.G.; Hopp, H.P.; Leslie, F.M.; Schell, M.J. The influence of a low-fat diet on incidence and severity of migraine headaches. J. Women’s Health Gender-Based Med. 1999, 8, 623–630.
- Ferrara, L.A.; Pacioni, D.; Di Fronzo, V.; Russo, B.F.; Speranza, E.; Carlino, V.; Gargiulo, F.; Ferrara, F. Low-lipid diet reduces frequency and severity of acute migraine attacks. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 370–375.
- Nowaczewska, M.; Wiciński, M.; Kaźmierczak, W.; Kaźmierczak, H. To eat or not to eat: A review of the relationship between chocolate and migraines. Nutrients 2020, 12, 608.
- Marcus, D.A.; Scharff, L.; Turk, D.C.; Gourley, L.M. A double-blind provocative study chocolate as a trigger of headache. Cephalalgia 1997, 17, 855–862.