Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
With the development of nanotechnology and confronting the problems of traditional pharmaceutical formulations in treating lung diseases, inhalable nano-formulations have attracted interest. Inhalable nano-formulations for treating lung diseases allow for precise pulmonary drug delivery, overcoming physiological barriers, improving aerosol lung deposition rates, and increasing drug bioavailability. They are expected to solve the difficulties faced in treating lung diseases.
- inhalable nano-formulations
- inhalation devices
- industrialization
- nebulizers
1. Introduction
Nano-formulations are diversely defined by different pharmaceutical scientists and drug regulatory agencies all over the world. The nano-formulations mentioned in this work refer to the nanoscale particles prepared from pure active pharmaceutical ingredients (APIs) using nano-formulation technology or nanoscale particles formed by combining APIs with appropriate carrier materials and the final pharmaceutical preparations thereof [1]. The final product or carrier material of nano-formulations has a particle size of typically less than 1000 nm, with significant scaling effects, and typically exhibits a well-defined physical interface [2].
The main types of nano-formulations are liposomes [3][4], polymer micelles [5][6], nanoemulsions [7][8], nanocrystals [9][10], etc. Compared with conventional formulations, nano-formulations may have the following potential: (i) increasing the solubility and bioavailability of the APIs or significantly reducing the food effect and inter-individual differences; (ii) increasing the stability of the APIs in vitro and in vivo; (iii) controlling the release profile of the APIs; (iv) improving the selectivity of the APIs to tissues, organs, or cells and thus enhancing the efficacy of the APIs and reducing adverse reactions; (v) offering new routes of drug delivery; and (vi) changing the physical status of the APIs. As a result, the convenience of clinical administration and the patient’s compliance can be elevated [2][11][12][13][14][15][16]. Nano-formulations are currently used in a variety of routes of administration, including intravenous [17], oral [18], transdermal [19], ocular [20], and pulmonary [21] delivery, for treating systemic and local diseases. In recent years, inhalable nano-formulations have generated a great deal of interest for the following reasons.
The global morbidity and mortality of respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma have increased from 1990 to the present [22][23][24][25][26]. Despite the progress in drug discovery and clinical diagnosis, there is still a lack of effective treatments for these diseases. Over the past 50 years, the incidence and mortality rates of lung cancer have increased significantly, ranking as the first place of all malignant tumors in males and second place in females. More and more attention is being paid to the pathogenesis, diagnosis, and treatment of lung cancer [27][28][29][30][31]. In addition, recent outbreaks of respiratory infectious diseases such as COVID-19 have accumulated global research interests [32][33][34][35][36]. The difficulty in treating these respiratory diseases may be due to inadequate doses of drugs entering the respiratory tract or insufficient targetability to the lesion sites when conventional pharmaceutical preparations are used [37][38].
In this context, there has been a focus on new approaches to achieve more effective treatment of lung diseases, in which inhalable nano-formulations have attracted the interest of many researchers with the development of nanotechnology. Inhalable nano-formulations have the following advantages in respiratory disease therapy: (i) reducing the administration dosage [39][40]; (ii) increasing the solubility of the APIs [41][42]; (iii) achieving targeted drug delivery towards lung lesions [37][43]; (iv) API absorption across the epithelium can be enhanced [44][45]; and (v) enabling pulmonary retention [46][47]. Due to these advantages, nanotechnology can ensure the therapeutic efficacy of APIs in dissatisfactory situations where the patient’s condition (e.g., unconsciousness, insufficient inspiratory flow rate, breath-holding problems, and inadequate coordination with the use of inhalation devices) results in poor inhalation effectiveness. Therefore, inhalable nano-formulations are considered to have promising applications in treating COPD, asthma, lung cancer, COVID-19, and other lung diseases [23][42][48][49].
Up till now, nano-formulations investigated for pulmonary drug delivery (summarized in Figure 1) mainly include polymeric nanocarriers (e.g., polymeric nanoparticles and polymeric micelles) [50][51], lipid-based nanocarriers (e.g., liposomes and solid lipid nanoparticles) [4][52], protein-based nanocarriers (e.g., albumin and engineered proteins) [53][54], inorganic nanocarriers (e.g., gold nanoparticles and calcium phosphate nanoparticles) [55][56], and biomimetic nanocarriers (e.g., cell membrane and exosomes) [42][57][58]. The researchers observe that nano-formulations are booming in the field of pulmonary delivery, and a diversity of nano-formulations can be regarded as drug candidates for respiratory disease treatment. Ultimately, nano-formulations are incorporated into inhalation devices (e.g., nebulizers, dry powder inhalers (DPIs), metered dose inhalers (MDIs)) for treating respiratory disease, either by themselves or with excipients to form solid particles [37].
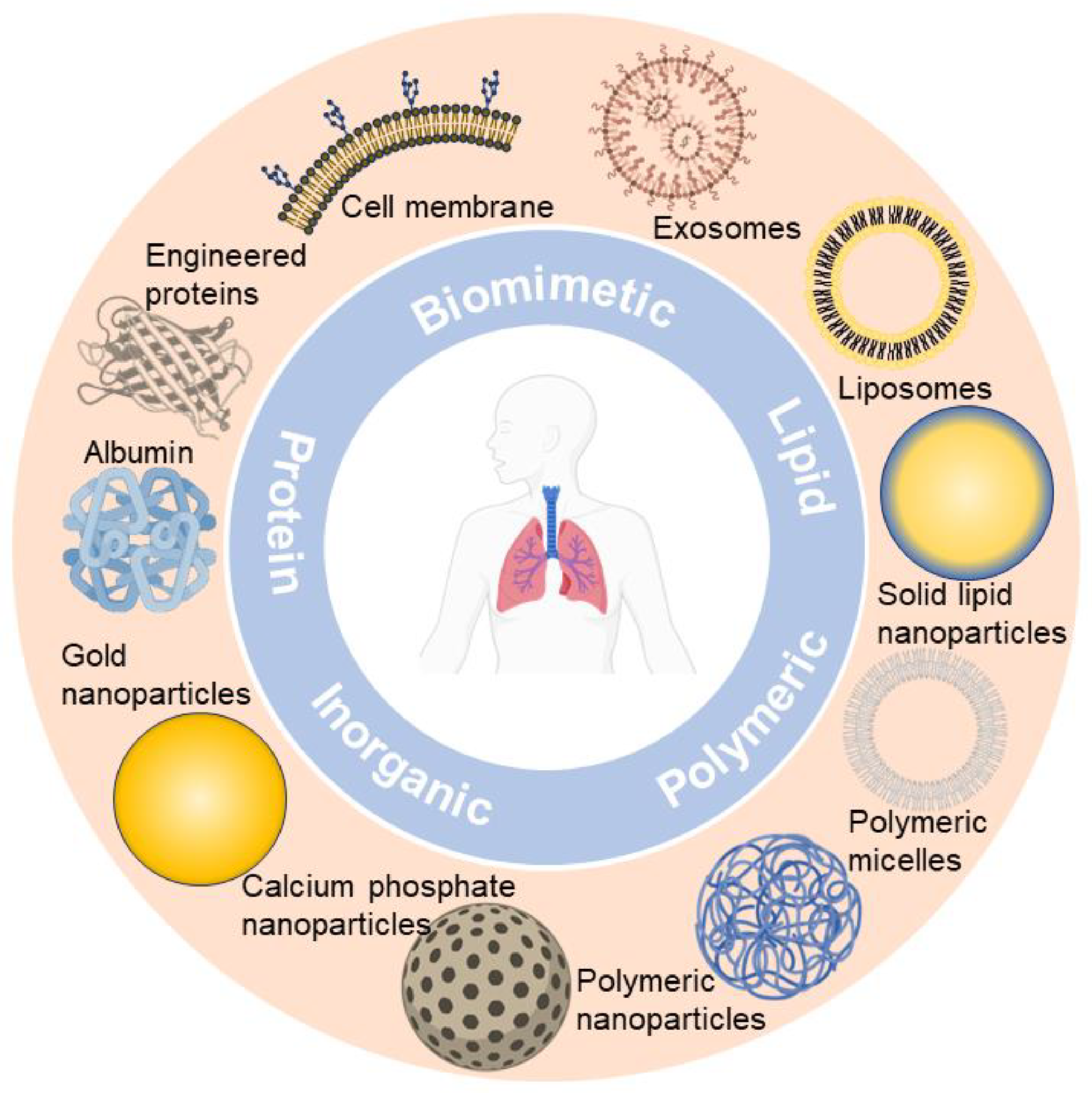
Figure 1. Representative nano-formulations in pulmonary drug delivery.
2. Fundamental Research on Inhalable Nano-Formulations
Regarding the current study of inhalable nano-formulations, the physicochemical, pharmaceutical, and toxicological properties have been intensively studied, which can be categorized into fundamental research. The firm knowledge acquired from fundamental research is the key basis of industrial translation. The well-documented fundamental research paradigm is depicted in Figure 2 and summarized as follows:
Figure 2. Fundamental research paradigm of inhalable nano-formulations.
- (i)
-
Synthesis methods. There are various synthesis methods available for different inhalable nano-formulations, which can be broadly categorized into top–down and bottom–up methods. Top–down methods refer to the decomposition of larger solid particles into smaller nanoparticles by external forces, such as high-pressure homogenization and wet milling. Bottom–up methods refer to the synthesis of nanoparticles from the molecular level by precipitation, crystallization, and the removal of solvents, such as extrusion, solvent evaporation, and antisolvent methods [4][59][60][61][62].
- (ii)
-
Structure elucidation. For synthesized nano-formulations, we need to determine the nanoarchitectonics, including the atomic, molecular, nanoscale, and mesoscale structures. Techniques such as ultraviolet spectrum, infrared spectrum, nuclear magnetic resonance spectroscopy, mass spectrometry, X-ray diffraction, and X-ray photoelectron spectroscopy can be utilized [63].
- (iii)
-
Size measurement. The size distribution of nano-formulations is commonly studied by nanoparticle size analyzers. The main tests include PDI, size distribution, and autocorrelation function (ACF) curves, to ensure that the size of the prepared nano-formulations meets the inhalation requirements. After nano-formulations have been prepared into forms suitable for use in inhalation devices such as DPIs, nebulizers, and others, it is equally necessary to study their particle size, PDI, and other properties, to ensure that the sprayed droplets or dry powders meet the size requirements for effective lung deposition [45][64][65].
- (iv)
-
Shape identification. The shape of nano-formulations is commonly studied using a Transmission Electron Microscope (TEM), a Scanning Electron Microscope (SEM), and an Atomic Force Microscope (AFM). In addition to the static shape, it is vital to study the shape of sprayed droplets or dry powders after administration to observe the corresponding changes. The shape of xenobiotic particles affects the physiological behavior. For instance, elongated or rod-shaped particles are difficult to be phagocytosed by macrophages, while spherical or elliptical particles have a stronger targeting effect on macrophages [66]. If we can better understand the interplay between the particle shape and the cells, it will help us to develop pulmonary drug delivery systems with greater targetability [67].
- (v)
-
Drug-carrying capacity. The large specific surface area or accommodation room of nanoparticles allows for the surface adsorption or physical encapsulation of drug molecules, leading to a better drug-carrying capacity compared to formulations with larger particles [68][69]. The drug-carrying capacity of nano-formulations is commonly analyzed by High-Performance Liquid Chromatography (HPLC) or HPLC tandem methods.
- (vi)
-
Release behavior. For the release profile of inhalable nano-formulations in the lungs, they are generally expected to endow a rapid and complete one after deposition in the lungs, thus improving bioavailability [70]. The release behavior of nano-formulations is often studied in vitro by simulating the in vivo environment (like stimulating lung fluid), and release curves are drawn for analysis.
- (vii)
-
Aerodynamic properties. It is generally believed that the optimal aerodynamic size range for inhalable particles is 1–3 μm, with which a satisfactory lung deposition can be achieved [64]. From this viewpoint, compared with nano-formulations, micron-sized formulations have superior aerodynamic properties, and thus, current studies seek to enlarge the aerodynamic diameter of the nano-formulations through microencapsulation or bulking techniques, without affecting the excellent bioavailability of the nano-formulations. The Next-Generation Impactor (NGI) is commonly used to study the aerodynamic properties of nano-formulations. Via the NGI, parameters such as fine particle dose (FPD), fine particle fraction (FPF), mass median aerodynamic particle diameter (MMAD), and geometric standard deviation (GSD) can be determined as indicators of aerodynamic performance [71][72].
- (viii)
-
Toxicity. The toxicity of nano-formulations mainly stems from two aspects, APIs and nanocarriers. In the area of pharmaceutics, nanocarrier toxicity is emphasized. Smaller nanocarriers are difficult to phagocytose by macrophages and are thus retained in the alveoli, which may produce side effects [73]. In addition, the residual organic solvents and metal ions remaining in the formulations may cause inflammatory and other adverse reactions [74][75]. We need to conduct in vitro and in vivo toxicity testing of the nano-formulations, to assess the safety [72]. Interestingly, it is pointed out that the precise targeting of the nano-formulations can prevent the non-specific interactions between nano-formulations and lung cells and thus alleviate local toxicity [76].
- (ix)
-
Pharmacokinetics. By improving the pulmonary deposition rate of nano-formulations as well as active targeting modifications, the pharmacokinetics properties may be enhanced [42]. High-Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS) is commonly used for pharmacokinetic-related studies. Inhalable nano-formulations in the lungs should be concerned with the absorption, distribution, metabolism, and excretion processes [37][70].
- (x)
-
Pharmacodynamics. The efficacy of nano-formulations can be enhanced by designing and screening the optimal formulation and administration scheme [42]. Pharmacodynamic studies of nano-formulations in pulmonary delivery are conducted in vitro to investigate whether the formulations produce the desired effects on cells (e.g., killing cancer cells, regulating gene expression and production of anti-inflammatory factors, etc.). In vivo, experiments were conducted to investigate whether the formulations could treat animals with lung diseases [28][40][45][49][77].
3. The Development of Inhalable Nano-Formulations and Their Nebulizers
As technology advances, more attention is being paid to inhalable nano-formulations, and nebulization technology continues to evolve to match the various new formulations. Although inhalable nano-formulations have many advantages over traditional formulations, and nebulizers are suitable as inhalation devices of nano-formulations, we must realize that only one inhaled nano-formulation has been marketed so far, which indicates that there are still many major problems in the development of inhalable nano-formulations and their nebulizers.
Firstly, regarding the safety of nanomaterials, we must consider improving the safety of the nanomaterials used in industrialization. Liposomes, as the first FDA-approved nanocarriers, are good in biocompatibility and safety. However, for cationic liposomes to deliver sensitive compounds such as nucleic acids, there is still the problem of cytotoxicity, which needs to be further addressed to improve their safety [78]. Dendritic polymers increase the number of cations by branching with a number of terminal amino groups. Excessive cations can lead to cell membrane rupture and apoptosis, producing severe cytotoxicity [78]. The non-degradability of inorganic nanocarriers leads to their continuous accumulation in the reticuloendothelial system, which triggers inflammation and other adverse reactions [42]. Overall, during the design of inhalable nano-formulations, we recommend the use of FDA-approved nanomaterials, which will ensure the safety of the formulation and increase the feasibility of translation. In addition to the safety of nanomaterials, we should pay attention to the in vivo retention and distribution processes of nano-formulations when conducting fundamental research. For example, the retention of nano-formulations in the lungs, the absorption rate in the pulmonary capillaries, and the systemic side effects due to increased systemic exposure should all be taken into consideration [62]. All of these issues must be examined in the industrialization of inhalable nano-formulations.
Secondly, in the nebulization of nano-formulations, changes in nebulization parameters and related nano-formulation parameters can affect the nebulization process [37]. Therefore, to ensure an effective deposition rate, reduce adverse effects, and improve bioavailability, we need to consider both the nebulizer parameters and the physicochemical properties of nano-formulations. Active vibrating mesh nebulizers have low shear stress and low API loss [79]. They are equipped with electronic controls such as eFlow® and AKITA® APIXNEB systems, which optimize the nebulization process by controlling the flow rate, output, and time of the aerosol [79][80]. Parameters such as the osmotic pressure, pH, and viscosity of the nano-formulations can also affect the nebulization process and effective deposition rate. Low osmotic pressure may cause adverse reactions such as coughing and bronchoconstriction. High osmotic pressure stimulates respiratory secretion and may aggravate respiratory symptoms. In addition, the improper application of excipients that regulate osmotic pressure may affect the therapeutic efficacy and safety [37][81][82]. A high or low pH may lead to the leakage of the drug and adverse reactions such as bronchospasm and coughing in patients [81][83]. Increased viscosity may increase the nebulized droplet size, prolong the nebulization time, and disrupt the drug structure, thus reducing the output efficacy and affecting nebulizer operation [84][85][86]. Overall, the design of inhalable nano-formulations involves the choice of a reasonable inhalation device and the adjustment of the relevant parameters with suitable excipients to optimize the nebulization process and improve the effective deposition rate.
Finally, in large-scale production, we need to simplify the design of nano-formulations, consider issues such as cost, industrial equipment, etc., and pay attention to the introduction of relevant standards [2][37][87]. ARIKAYCE®, as mentioned above, is simple in design and production. The Lamira® nebulization system used was modified for large-scale production. In addition, the online quality control equipment and some of the welding techniques were customized [79][88]. The industrialization of inhalable nano-formulations is also dependent on the formulation of standards by governmental departments and the unification of global standards. This will guide and promote researchers to carry out industrialized research on inhalable nano-formulations and achieve the purpose of large-scale production.
In conclusion, the development of inhalable nano-formulations and their nebulizers requires a detailed and feasible plan designed ab initio, and this plan needs to be continuously improved to meet the requirements for translation. Finally, the challenges may be gradually resolved to achieve large-scale production, through the collaboration of various fields.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics16020161
References
- Food and Drug Administration. Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology Contains Nonbinding Recommendations; Food and Drug Administration: Silver Spring, MD, USA, 2014.
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Nano-Formulations of Drugs: Recent Developments, Impact and Challenges. Biochimie 2016, 128–129, 99–112.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102.
- Has, C.; Sunthar, P. A Comprehensive Review on Recent Preparation Techniques of Liposomes. J. Liposome Res. 2020, 30, 336–365.
- Jones, M.-C.; Leroux, J.-C. Polymeric Micelles—A New Generation of Colloidal Drug Carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111.
- Kwon, G.S. Polymeric Micelles as New Drug Carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116.
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Control. Release 2017, 252, 28–49.
- Sarker, D. Engineering of Nanoemulsions for Drug Delivery. Curr. Drug Deliv. 2005, 2, 297–310.
- Pardhi, V.P.; Verma, T.; Flora, S.J.S.; Chandasana, H.; Shukla, R. Nanocrystals: An Overview of Fabrication, Characterization and Therapeutic Applications in Drug Delivery. Curr. Pharm. Des. 2018, 24, 5129–5146.
- Shi, J.; Guo, F.; Zheng, A.; Zhang, X.; Sun, J. Progress in the Study of Drug Nanocrystals. Pharmazie 2015, 70, 757–764.
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent Advances in Nanoparticle-Based Cancer Drug and Gene Delivery. Adv. Cancer Res. 2018, 137, 115–170.
- Singh, R.; Lillard, J.W. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215–223.
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021, 16, 1313–1330.
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79.
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-Formulations for Transdermal Drug Delivery: A Review. Chin. Chem. Lett. 2018, 29, 1713–1724.
- Xing, Y.; Lu, P.; Xue, Z.; Liang, C.; Zhang, B.; Kebebe, D.; Liu, H.; Liu, Z. Nano-Strategies for Improving the Bioavailability of Inhaled Pharmaceutical Formulations. Mini-Rev. Med. Chem. 2020, 20, 1258–1271.
- Porche, D.J. Liposomal Doxorubicin (Doxil®). J. Assoc. Nurses AIDS Care 1996, 7, 55–59.
- Caldwell, J.R. Avinza®—24-h Sustained-Release Oral Morphine Therapy. Expert. Opin. Pharmacother. 2004, 5, 469–472.
- Chiechi, L.M. Estrasorb. IDrugs 2004, 7, 860–864.
- Mandal, A.; Gote, V.; Pal, D.; Ogundele, A.; Mitra, A.K. Ocular Pharmacokinetics of a Topical Ophthalmic Nanomicellar Solution of Cyclosporine (Cequa®) for Dry Eye Disease. Pharm. Res. 2019, 36, 36.
- Khan, O.; Chaudary, N. The Use of Amikacin Liposome Inhalation Suspension (Arikayce) in the Treatment of Refractory Nontuberculous Mycobacterial Lung Disease in Adults. Drug Des. Devel Ther. 2020, 14, 2287–2294.
- Soriano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Aichour, M.T.E.; Alam, K.; et al. Global, Regional, and National Deaths, Prevalence, Disability-Adjusted Life Years, and Years Lived with Disability for Chronic Obstructive Pulmonary Disease and Asthma, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706.
- Forno, E.; Ortega, V.E.; Celedón, J.C. Asthma and Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2023, 44, 519–530.
- Mims, J.W. Asthma: Definitions and Pathophysiology. Int. Forum Allergy Rhinol. 2015, 5, S2–S6.
- Rabe, K.F.; Watz, H. Chronic Obstructive Pulmonary Disease. Lancet 2017, 389, 1931–1940.
- Wu, F.; Deng, Z.S.; Tian, H.S.; Li, H.Q.; Zhou, Y.M. . Zhonghua Jie He He Hu Xi Za Zhi 2023, 46, 1028–1034.
- Mao, Y.; Yang, D.; He, J.; Krasna, M.J. Epidemiology of Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 439–445.
- Nasim, F.; Sabath, B.F.; Eapen, G.A. Lung Cancer. Med. Clin. N. Am. 2019, 103, 463–473.
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24.
- Oliver, A.L. Lung Cancer: Epidemiology and Screening. Surg. Clin. N. Am. 2022, 102, 335–344.
- Abu Rous, F.; Singhi, E.K.; Sridhar, A.; Faisal, M.S.; Desai, A. Lung Cancer Treatment Advances in 2022. Cancer Investig. 2023, 41, 12–24.
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 Vaccine Development Landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306.
- Shi, Y.; Wang, G.; Cai, X.; Deng, J.; Zheng, L.; Zhu, H.; Zheng, M.; Yang, B.; Chen, Z. An Overview of COVID-19. J. Zhejiang Univ. Sci. B 2020, 21, 343–360.
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 128.
- Ochani, R.; Asad, A.; Yasmin, F.; Shaikh, S.; Khalid, H.; Batra, S.; Sohail, M.R.; Mahmood, S.F.; Ochani, R.; Hussham Arshad, M.; et al. COVID-19 Pandemic: From Origins to Outcomes. A Comprehensive Review of Viral Pathogenesis, Clinical Manifestations, Diagnostic Evaluation, and Management. Infez. Med. 2021, 29, 20–36.
- Yuan, Y.; Jiao, B.; Qu, L.; Yang, D.; Liu, R. The Development of COVID-19 Treatment. Front. Immunol. 2023, 14, 1125246.
- Forest, V.; Pourchez, J. Nano-Delivery to the Lung—By Inhalation or Other Routes and Why Nano When Micro Is Largely Sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173.
- Zhou, Q.T.; Leung, S.S.Y.; Tang, P.; Parumasivam, T.; Loh, Z.H.; Chan, H.-K. Inhaled Formulations and Pulmonary Drug Delivery Systems for Respiratory Infections. Adv. Drug Deliv. Rev. 2015, 85, 83–99.
- Joshi, K.; Chandra, A.; Jain, K.; Talegaonkar, S. Nanocrystalization: An Emerging Technology to Enhance the Bioavailability of Poorly Soluble Drugs. Pharm. Nanotechnol. 2019, 7, 259–278.
- Akhter, S.; Ahmad, J.; Rizwanullah, M.; Rahman, M.; Zaki Ahmad, M.; Rizvi, M.M.A.; Ahmad, F.J.; Amin, S.; Kamal, M.A. Nanotechnology-Based Inhalation Treatments for Lung Cancer: State of the Art. Nanotechnol. Sci. Appl. 2015, 8, 55–66.
- Ngan, C.L.; Asmawi, A.A. Lipid-Based Pulmonary Delivery System: A Review and Future Considerations of Formulation Strategies and Limitations. Drug Deliv. Transl. Res. 2018, 8, 1527–1544.
- Jin, Z.; Gao, Q.; Wu, K.; Ouyang, J.; Guo, W.; Liang, X.-J. Harnessing Inhaled Nanoparticles to Overcome the Pulmonary Barrier for Respiratory Disease Therapy. Adv. Drug Deliv. Rev. 2023, 202, 115111.
- Wijagkanalan, W.; Kawakami, S.; Takenaga, M.; Igarashi, R.; Yamashita, F.; Hashida, M. Efficient Targeting to Alveolar Macrophages by Intratracheal Administration of Mannosylated Liposomes in Rats. J. Control. Release 2008, 125, 121–130.
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951.
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for Drug Delivery to the Lungs. Trends Biotechnol. 2007, 25, 563–570.
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003.
- Yang, Y.; Liu, X.; Ma, W.; Xu, Q.; Chen, G.; Wang, Y.; Xiao, H.; Li, N.; Liang, X.-J.; Yu, M.; et al. Light-Activatable Liposomes for Repetitive on-Demand Drug Release and Immunopotentiation in Hypoxic Tumor Therapy. Biomaterials 2021, 265, 120456.
- Horejs, C. Nebulized Lipid Nanoparticles. Nat. Rev. Mater. 2021, 6, 1077.
- Zarogoulidis, P.; Chatzaki, E.; Porpodis, K.; Domvri, K.; Hohenforst-Schmidt, W.; Goldberg, E.P.; Karamanos, N.; Zarogoulidis, K. Inhaled Chemotherapy in Lung Cancer: Future Concept of Nanomedicine. Int. J. Nanomed. 2012, 7, 1551–1572.
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663.
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric Micelles for Nano-Scale Drug Delivery. React. Funct. Polym. 2011, 71, 227–234.
- Videira, M.; Almeida, A.J.; Fabra, À. Preclinical Evaluation of a Pulmonary Delivered Paclitaxel-Loaded Lipid Nanocarrier Antitumor Effect. Nanomedicine 2012, 8, 1208–1215.
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387.
- Pápay, Z.E.; Kósa, A.; Böddi, B.; Merchant, Z.; Saleem, I.Y.; Zariwala, M.G.; Klebovich, I.; Somavarapu, S.; Antal, I. Study on the Pulmonary Delivery System of Apigenin-Loaded Albumin Nanocarriers with Antioxidant Activity. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 274–288.
- Chen, Y.-H.; Tsai, C.-Y.; Huang, P.-Y.; Chang, M.-Y.; Cheng, P.-C.; Chou, C.-H.; Chen, D.-H.; Wang, C.-R.; Shiau, A.-L.; Wu, C.-L. Methotrexate Conjugated to Gold Nanoparticles Inhibits Tumor Growth in a Syngeneic Lung Tumor Model. Mol. Pharm. 2007, 4, 713–722.
- Quarta, E.; Degli Esposti, L.; Bettini, R.; Sonvico, F.; Catalucci, D.; Iafisco, M.; De Luca, C.; Trevisi, G.; Colombo, P.; Rossi, A.; et al. Dry Powder Inhalation Technology for Heart Targeting Applied to Calcium Phosphate Nanoparticles Loaded with Active Substances. J. Drug Deliv. Sci. Technol. 2022, 76, 103719.
- Dash, P.; Piras, A.M.; Dash, M. Cell Membrane Coated Nanocarriers—An Efficient Biomimetic Platform for Targeted Therapy. J. Control. Release 2020, 327, 546–570.
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent Advancements in the Use of Exosomes as Drug Delivery Systems. J. Nanobiotechnology 2018, 16, 81.
- Taratula, O.; Kuzmov, A.; Shah, M.; Garbuzenko, O.B.; Minko, T. Nanostructured Lipid Carriers as Multifunctional Nanomedicine Platform for Pulmonary Co-Delivery of Anticancer Drugs and SiRNA. J. Control. Release 2013, 171, 349–357.
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and Innovation in the Manufacturing Process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122.
- Sabjan, K.B.; Munawar, S.M.; Rajendiran, D.; Vinoji, S.K.; Kasinathan, K. Nanoemulsion as Oral Drug Delivery—A Review. Curr. Drug Res. Rev. 2020, 12, 4–15.
- Zhang, J.; Wu, L.; Chan, H.-K.; Watanabe, W. Formation, Characterization, and Fate of Inhaled Drug Nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 441–455.
- Negi, L.M.; Jaggi, M.; Talegaonkar, S. Development of Protocol for Screening the Formulation Components and the Assessment of Common Quality Problems of Nano-Structured Lipid Carriers. Int. J. Pharm. 2014, 461, 403–410.
- Lavorini, F.; Buttini, F.; Usmani, O.S. 100 Years of Drug Delivery to the Lungs. Concepts Princ. Pharmacol. 2019, 260, 143–159.
- Wang, W.; Huang, Z.; Li, Y.; Wang, W.; Shi, J.; Fu, F.; Huang, Y.; Pan, X.; Wu, C. Impact of Particle Size and PH on Protein Corona Formation of Solid Lipid Nanoparticles: A Proof-of-Concept Study. Acta Pharm. Sin. B 2021, 11, 1030–1046.
- Champion, J.A.; Mitragotri, S. Shape Induced Inhibition of Phagocytosis of Polymer Particles. Pharm. Res. 2009, 26, 244–249.
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The Importance of Nanoparticle Shape in Cancer Drug Delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142.
- Bailey, M.M.; Berkland, C.J. Nanoparticle Formulations in Pulmonary Drug Delivery. Med. Res. Rev. 2009, 29, 196–212.
- Loira-Pastoriza, C.; Todoroff, J.; Vanbever, R. Delivery Strategies for Sustained Drug Release in the Lungs. Adv. Drug Deliv. Rev. 2014, 75, 81–91.
- Ibarra-Sánchez, L.Á.; Gámez-Méndez, A.; Martínez-Ruiz, M.; Nájera-Martínez, E.F.; Morales-Flores, B.A.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Iqbal, H.M.N. Nanostructures for Drug Delivery in Respiratory Diseases Therapeutics: Revision of Current Trends and Its Comparative Analysis. J. Drug Deliv. Sci. Technol. 2022, 70, 103219.
- Harush-Frenkel, O.; Bivas-Benita, M.; Nassar, T.; Springer, C.; Sherman, Y.; Avital, A.; Altschuler, Y.; Borlak, J.; Benita, S. A Safety and Tolerability Study of Differently-Charged Nanoparticles for Local Pulmonary Drug Delivery. Toxicol. Appl. Pharmacol. 2010, 246, 83–90.
- Keil, T.W.M.; Feldmann, D.P.; Costabile, G.; Zhong, Q.; da Rocha, S.; Merkel, O.M. Characterization of Spray Dried Powders with Nucleic Acid-Containing PEI Nanoparticles. Eur. J. Pharm. Biopharm. 2019, 143, 61–69.
- Mansour, H.M.; Rhee, Y.S.; Wu, X. Nanomedicine in Pulmonary Delivery. Int. J. Nanomed. 2009, 4, 299–319.
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual Polyvinyl Alcohol Associated with Poly (d,l-Lactide-Co-Glycolide) Nanoparticles Affects Their Physical Properties and Cellular Uptake. J. Control. Release 2002, 82, 105–114.
- Cho, W.-S.; Duffin, R.; Poland, C.A.; Duschl, A.; Oostingh, G.J.; MacNee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Differential Pro-Inflammatory Effects of Metal Oxide Nanoparticles and Their Soluble Ions In Vitro and In Vivo; Zinc and Copper Nanoparticles, but Not Their Ions, Recruit Eosinophils to the Lungs. Nanotoxicology 2012, 6, 22–35.
- Kaminskas, L.M.; McLeod, V.M.; Ryan, G.M.; Kelly, B.D.; Haynes, J.M.; Williamson, M.; Thienthong, N.; Owen, D.J.; Porter, C.J.H. Pulmonary Administration of a Doxorubicin-Conjugated Dendrimer Enhances Drug Exposure to Lung Metastases and Improves Cancer Therapy. J. Control. Release 2014, 183, 18–26.
- Couvreur, P. Nanoparticles in Drug Delivery: Past, Present and Future. Adv. Drug Deliv. Rev. 2013, 65, 21–23.
- Wang, R.; Zhang, X.; Feng, K.; Zeng, W.; Wu, J.; Sun, D.; Lu, Z.; Feng, H.; Di, L. Nanotechnologies Meeting Natural Sources: Engineered Lipoproteins for Precise Brain Disease Theranostics. Asian J. Pharm. Sci. 2023, 18, 100857.
- Lass, J.S.; Sant, A.; Knoch, M. New Advances in Aerosolised Drug Delivery: Vibrating Membrane Nebuliser Technology. Expert Opin. Drug Deliv. 2006, 3, 693–702.
- Zhou, Q.T.; Tang, P.; Leung, S.S.Y.; Chan, J.G.Y.; Chan, H.-K. Emerging Inhalation Aerosol Devices and Strategies: Where Are We Headed? Adv. Drug Deliv. Rev. 2014, 75, 3–17.
- Schoni, M.; Kraemer, R. Osmolality Changes in Nebulizer Solutions. Eur. Respir. J. 1989, 2, 887–892.
- Eschenbacher, W.L.; Boushey, H.A.; Sheppard, D. Alteration in Osmolarity of Inhaled Aerosols Cause Bronchoconstriction and Cough, but Absence of a Permeant Anion Causes Cough Alone. Am. Rev. Respir. Dis. 1984, 129, 211–215.
- Niven, R.W.; Carvajal, T.M.; Schreier, H. Nebulization of Liposomes. III. The Effects of Operating Conditions and Local Environment. Pharm. Res. 1992, 09, 515–520.
- Ghazanfari, T.; Elhissi, A.M.A.; Ding, Z.; Taylor, K.M.G. The Influence of Fluid Physicochemical Properties on Vibrating-Mesh Nebulization. Int. J. Pharm. 2007, 339, 103–111.
- Mc Callion, O.N.M.; Patel, M.J. Viscosity Effects on Nebulisation of Aqueous Solutions. Int. J. Pharm. 1996, 130, 245–249.
- Weber, A.; Morlin, G.; Cohen, M.; Williams-Warren, J.; Ramsey, B.; Smith, A. Effect of Nebulizer Type and Antibiotic Concentration on Device Performance. Pediatr. Pulmonol. 1997, 23, 249–260.
- Yohan, D.; Chithrani, B.D. Applications of Nanoparticles in Nanomedicine. J. Biomed. Nanotechnol. 2014, 10, 2371–2392.
- Li, Z.; Perkins, W.; Cipolla, D. Robustness of Aerosol Delivery of Amikacin Liposome Inhalation Suspension Using the EFlow® Technology. Eur. J. Pharm. Biopharm. 2021, 166, 10–18.
This entry is offline, you can click here to edit this entry!
