Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Measures to endorse the adoption of eco-friendly biodegradable plastics as a response to the scale of plastic pollution have created a demand for innovative products from materials from Nature. Ionic liquids (ILs) have the ability to disrupt the hydrogen bonding network of biopolymers, increase the mobility of biopolymer chains, reduce friction, and produce materials with various morphologies and mechanical properties. Due to these qualities, ILs are considered ideal for plasticizing biopolymers, enabling them to meet a wide range of specifications for biopolymeric materials.
- biopolymers
- materials
- plasticizer
- ionic liquids
1. Introduction
With the increase in the quantity of synthetic plastic, the damaging effects of plastic waste on the environment have also intensified, and the scale of worldwide plastic pollution has become one of the most persistent public concerns. The underlying reasons for this are the affordability, convenience, and accessibility of synthetic plastics. Of the 6300 metric tons (mt) of plastics discarded in 2015, ~550 mt (~9%) have been recycled, ~750 mt (~12%) incinerated, and as much as ~5000 mt have been accumulated in the environment [1]. With the current production and recycling rate of plastics, ~12,000 mt of plastics is expected to accumulate by 2050 [1]. Emphasizing the interest in polymer degradability, several excellent comprehensive review articles on biodegradable polymers have been published and have critically emphasized their effective use in various areas, including packaging (e.g., coating films, food containers, wrapping), agriculture (e.g., mulching films), and biomedical/biotechnology (e.g., tissue engineering scaffolds, drug and gene delivery matrices, wound healing hydrogels, dental materials) [2,3,4,5,6].
After realizing the environmental impact of fuel-based plastics, many governments have instigated rigorous measures to endorse the notion of creating materials from renewable resources and to increase the adoption of eco-friendly biodegradable plastics from Nature-based materials. These materials are designed to biodegrade in the natural environment over time, reducing the long-term environmental impact of plastic waste. In addition to initiatives taken by governments and global companies, there is also a growing awareness from consumers who are paying attention to the global climate crisis. Furthermore, the circular economy action plan was endorsed by leaders from the World Economic Forum, the European Parliament, and Fortune 500 companies as a tactic to restore the environment [7]. A circular economy is restorative by intention [8] and aims for “the elimination of waste through the strategic design of materials, products, and processes”. This leads to finding suitable sustainable solutions, preferentially from renewable feedstocks. Waste streams represent an even better alternative, as their usage allows us to fully remove waste from the industrial chain [9].
2. Ionic Liquids and Biopolymers
Ionic Liquids (ILs) are loosely defined as salts that melt below 100 °C [33]. Unlike conventional organic compounds, many of them exhibit negligible volatility, non-flammability, high thermal stability, and high conductivity. Since the discovery of cellulose dissolution in ILs [34], it has been shown that ILs are excellent solvents for the dissolution of a wide range of biopolymers comprising carbohydrates, proteins, and enzymatically produced polymers. Given the ability of ILs to dissolve virtually every renewable biomass to some extent, this capability results in the perfect platform for the preparation of high-value products for new and existing industrial applications that will substitute current plastics. This would result in new uses of unmodified biopolymers in technologies that currently use synthetic polymers, rather than breaking them down into platform chemicals. The research in this area takes advantage of the IL-based strategy, which avoids chemical transformations and allows the utilization of the existing functions and properties of the biopolymers “as is” in virtually any architecture.
Using ILs, biopolymers can be cast, molded, spun (using dry-/wet-jet extrusion) or electrospun, 3D printed, etc. to produce functional materials [35,36]. The addition of new functionality by forming composites with organic or inorganic solutes, nanoparticles, etc., is also possible during this process and further flexibility is available through the ability to produce biopolymer composites with tunable properties by blending them with other polymers. Finally, due to the insolubility of biopolymers in most solvents, they can be surface-modified through either covalent or ionic functionalization. Materials already made from biopolymers using this approach include hydrogels for drug delivery, chitin-calcium alginate composite fibers for wound care, electrospun chitin nanomaterials with specific chemical functionality such as catalysts, sorbents, filters, or sensors, films for drug delivery, and beads for water purification, to name a few [35,37].
Ion constituents of the IL affect the inter- and intra-molecular interactions in biopolymeric systems. Because anions in the ILs act as hydrogen bond acceptors, upon dissolution of the biopolymer, the hydrogen bonding network of biopolymers naturally present in these systems is disrupted and new bonds are formed between the anion of the IL and the OH- groups of the polymer (Figure 1). Simultaneously, the cation associates with the ether oxygen atoms or -CH group of the biopolymer [34,38,39]. It is known that biopolymeric solutions with the same polymer load are able to produce materials with different topological and mechanical properties upon dissolution/regeneration [40]. The reasons behind this are the steric and electronic effects of anions (e.g., bulkier [MeSO3]− vs. small Cl−) and cations (e.g., longer alkyl chain butyl- vs. shorter ethyl-), resulting in different interaction strengths between IL ions and biopolymers. For instance, the cellulose-silk films prepared using 1-allyl-3-methylimidazolium chloride ([Amim]Cl), 1-ethyl-3-methylimidazolium chloride ([C2mim]Cl), 1-butyl-3-methylimidazolium chloride ([C4mim]Cl), or 1-ethyl-3-methylimidazolium acetate ([C2mim][OAc]) are clearer, stronger, and less brittle than the same films prepared from 1-butyl-3-methylimidazolium bromide ([C4mim]Br) or 1-butyl-3-methylimidazolium methanesulfonate ([C4mim][MeSO3]) [40].
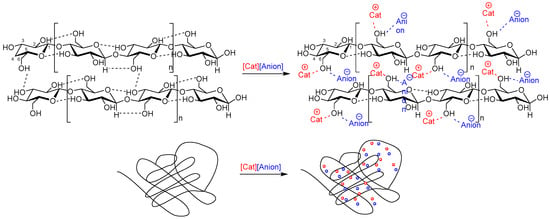
Figure 1. Intermolecular interactions between the components of an ionic liquid and cellulose as an example of a biopolymer.
Morphological changes based on IL are also evident and a larger extent of IL interactions with natural polymers causes more significant changes in crystallinity. The films regenerated from [Amim]Cl, [C2mim]Cl, and [C4mim]Cl were smooth, while the film made using [C2mim][OAc] possessed a somewhat porous structure caused by the formation of channels during the IL-removal process. The films regenerated from [C4mim]Br and [C4mim][MeSO3] had a fibrous string-like morphology. Respectively, the crystallinity of the material is also a function of the IL [40]. As determined by crystal fraction calculation (using FTIR spectra deconvolution and analysis of amide I absorbance band), the films prepared with chloride anion-containing ILs were the lowest in crystallinity. Thus, β-sheet crystal fraction for the films made using [Amim]Cl was 31.0%, [C4mim]Cl—37.5%, [C2mim]Cl—37.1% whereas bromide and methanesulfonate-containing ILs showed much higher β-sheet crystal fraction: [C4mim]Br—58.6%, [C4mim][MeSO3]—58.9%). These differences are correlated with the extent of disruption to the hydrogen bonding network. The X-ray scattering study confirmed that the prepared films were either amorphous or semicrystalline, and the spacing differences in the ILs correlated with the extent of intermolecular interactions.
3. Ionic Liquids as Plasticizers
From the above, it is evident that ILs can disrupt a hydrogen-bonding network of biopolymers, which makes them suitable to act as plasticizers. In fact, the ILs most reported as plasticizers are also reported to dissolve biopolymers (Figure 2). The plasticization and dissolution of biopolymers by ILs proceeds by the same mechanism, namely, the disruption of the biopolymer’s hydrogen bonding network [41,42,43,44,45,46,47].
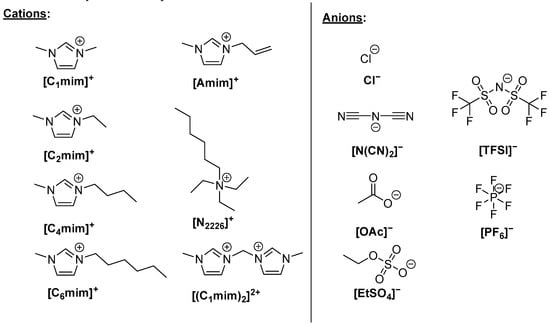
Figure 2. Ionic liquid ions commonly reported in the literature as plasticizers.
The primary property of ILs that can be used to understand this phenomenon is the ILs’ polarity [48]. The empirical solvent descriptors (often called coefficients, derived from the Kamlet–Taft equation) α- and β-, where α- is hydrogen bond acidity [49] and β- is hydrogen bond basicity [50,51], can provide a quantitative comparison between ILs. It was found that the basicity of the IL-anion controls β-coefficient whereas the IL “as a whole entity” controls the α-coefficient. For the full disruption of the hydrogen bonding network (i.e., for complete biopolymer dissolution), the ILs are required to have β-values > 0.8 (e.g., β > 0.5 is required for chitin dissolution, and β > 0.8 is required for cellulose dissolution) [52,53,54]. β-values increase when the anion has a hydrogen-bonding acceptor with a high electron density [55]. Hence, anion–cellulose interactions decrease in the order Cl− > [OAc]− > [(CH3O)2PO2]− > [SCN]− > [PF6]−. While (lower) β-values control the dissolution and plasticization of the biopolymers, indicating the greater importance of anions [56], α-values appear not to be as important. Although the exact mechanism of how cations are involved in the disruption of the hydrogen bonding network of the biopolymer is still under discussion [41,57,58], the cations also play a significant role in this process [59]. Molecular dynamics (MD) simulations confirmed these findings [60,61] and suggested that, in the presence of IL, hydrogen bonding interactions between the anion and the biopolymer arise, with the anions strongly interacting with the polar domains of the biopolymer. In contrast, the [Cnmim]+ cations interact with the nonpolar domains of the biopolymer via dispersion forces.
In addition to a high degree of compatibility with biopolymers and the ability to affect the morphology and crystallinity of the materials, many ILs demonstrate negligible vapor pressure, which is important since plasticizers should not evaporate from a bioplastic material, otherwise they will revert to their original brittle condition. This also results in reduced human exposure through evaporation. Many ILs exhibit low-temperature lubricity, high-temperature stability, enhanced stability to UV light, and reduced flammability [62]. In addition, the viscosity of the ILs can be tuned, an important property for ILs when they are used as plasticizers. Since ILs have a higher viscosity than conventional organic solvents due to hydrogen bonding and van der Waals interactions within the liquid, they make more preferential low-leaching plasticizers than organic compounds because of limited leaching and migration. To prepare ILs with the required viscosity (which depends on the polymer being plasticized), different strategies have been used, such as modifying the alkyl chain length of the cation, using different cationic cores, and changing the anion of the ILs [63]. The choice of the specific IL and its concentration can be tailored to achieve biopolymeric materials with specific properties, such as improved elasticity and reduced brittleness. This task involves adjusting both the type and concentration of the ionic liquid, as well as exploring different processing techniques to achieve the desired properties in the resulting biopolymer-based materials. As plasticizers, the IL-modified biopolymeric materials show changes in mechanical properties, i.e., a decrease in tensile strength and an increase in elongation at break are generally observed (Table 1).
Table 1. Examples of ILs used for plasticization of biopolymers and their effect on mechanical properties of the resulting materials.
| Biopolymer(s) or Derivative—Additive | Ionic Liquid | Tensile Strength (MPa) | Elongation at Break (%) | Ref. | ||
|---|---|---|---|---|---|---|
| Without IL | With IL | Without IL | With IL | |||
| Starch-zein | [C4mim]Cl | 1.38 ± 0.45 | 8.93 ± 1.59 | 8.06 ± 0.05 | 29.53 ± 0.86 | [64] |
| Starch | Polymeric IL | 10.37 ± 0.5 a | 4.91 ± 1 a | 2.25 ± 0.05 a | 6.81 ± 0.1 a | [65] |
| Starch | [C4mim]Cl | 1.9 ± 0.1 b | 0.6 ± 0.1 | 88 ± 7 b | 392 ± 27 | [65] |
| Starch | [C4mim][SO4Et] | ND c | 70± 8 | ND c | 35 ± 4 | [66] |
| Starch | [C4mim][OAc] | ND c | 53 ± 8 | ND c | 42 ± 4 | [66] |
| Starch | [C4mim][N(CN2)] | ND c | 45 ± 4 | ND c | 40 ± 3 | [66] |
| Starch | [C4mim]Cl | ND c | 14 ± 1 | ND c | 22 ± 1 | [66] |
| Starch | [(C1mim)2]Cl2 | ND c | 1600 ± 400 | ND c | 4 ± 1 | [66] |
| Chitosan-GO d | [C2mim][OAc] | 33 ± 3 a,b | 28 ± 2 a | 163 ± 16 a,b | 225 ± 25 a | [67] |
| Chitosan-rGO e | [C2mim][OAc] | 33 ± 2 a,b | 33 ± 3 a | 155 ± 12 a,b | 285 ± 20 a | [67] |
| Chitosan-alginate (50/50)-GO d | [C2mim][OAc] | 20 ± 1 a,b | 28 ± 4 a | 62 ± 1 a,b | 75 ± 3 a,b | [67] |
| Chitosan-alginate (50/50)-rGO e | [C2mim][OAc] | 20 ± 1 a,b | 21 ± 3 a | 62 ± 3 a,b | 50 ± 8 a,b | [67] |
| Chitosan-clay composite films | [C4mim]Cl | 12 ± 2 | 5.2 ± 1.1 | 1.5 ± 0.2 | 3.1 ± 1.2 | [68] |
| Chitosan | [C2mim][OAc] | 27 ± 2 b | 28 ± 2 a,f/12 ± 2 a,g | 72 ± 8 a,b | 70 ± 8 a,f/82 ± 12 a,g | [69,70] |
| Chitosan-carboxymethylcellulose | [C2mim][OAc] | 38 ± 3 a,b | 38 ± 3 a,f/23 ± 8 a,g | 70 ± 10 a,b | 35 ± 10 a,f/92 ± 20 a,g | [67,69] |
| Chitosan-carboxymethylcellulose (50/50)-GO d | [C2mim][OAc] | 33 ± 3 a,b | 37 ± 3 a,f/17 ± 4 a,g | 63 ± 10 a,b | 22 ± 12 a,f/110 ± 6 a,g | [67,69] |
| Chitosan-carboxymethylcellulose (50/50)-rGO e | [C2mim][OAc] | 34 ± 3 a,b | 40 ± 3 a,f/16 ± 4 a,g | 60 ± 10 a,b | 38 ± 11 a,f/100 ± 20 a,g | [67,69] |
| Chitosan-Sepolite | [C2mim][OAc] | 43 ± 1 a | 27 ± 1 a | 15 ± 1 a | 65 ± 15 a | [71] |
| Chitosan-CNC h | [C2mim][OAc] | 54 ± 2 a | 25 ± 2 a | 15 ± 2 a | 52 ± 8 a | [70] |
| Chitosan-Sepolite-CNC h | [C2mim][OAc] | 56 ± 0.5 a | 25 ± 2 a | 14 ± 2 a | 64 ± 11 a | [70] |
| Chitosan-carboxymethylcellulose (50/50)-Sepolite | [C2mim][OAc] | 57 ± 3 a | 37 ± 2 a | 20 ± 2 a | 30 ± 6 a | [70] |
| Chitosan-carboxymethylcellulose (50/50)-CNC h | [C2mim][OAc] | 57 ± 2 a | 36 ± 2 a | 22 ± 8 a | 34 ± 10 a | [70] |
| Chitosan-carboxymethylcellulose (50/50)-Sepolite-CNC h | [C2mim][OAc] | 56 ± 2 a | 46 ± 2 a | 24 ± 6 a | 35 ± 8 a | [70] |
| Cellulose triacetate | [C6mim][OAc] | 17.5 ± 2 a,i | 20 ± 1 a,i | 1.5 ± 0.1 a,i | 3 ± 0.1 a,i | [72] |
| Cellulose triacetate | [C6mim][PF6] | 17.5 ± 2 a,i | 12.5 ± 2 a,i | 1.5 ± 0.1 a,i | 15 ± 0.1 a,i | [72] |
| Cellulose triacetate | [N2226][OAc] | 17.5 ± 2 a,i | 15 ± 2 a,i | 1.5 ± 0.1 a,i | 2 ± 0.1 a,i | [72] |
| Cellulose triacetate | [N2226][PF6] | 17.5 ± 2 a,i | 8 ± 2 a,i | 1.5 ± 0.1 a,i | 11 ± 0.1 a,i | [72] |
| Cellulose acetate | [C4mim]Cl | 18.2 ± 0.1 a | 9 ± 2 a | 11.9 ± 2 a | 6.5 ± 0.2 a | [73] |
| Cellulose | [C4mim][TFSI] k | 41.5 j | 29 | ND | ND | [74] |
a Data extracted from graphs; b using glycerol as a plasticizer (20%); c due to the brittleness of the native starch film, it was not possible to perform tensile tests; d graphene oxide (GO); e reduced GO (rGO); f plasticized with 20% IL; g plasticized with 40% IL; h cellulose nanocrystals (CNC); i after 120 h; j plasticized with glycerin; k TFSI: bistriflimide or triflimidate anion, with the chemical formula [(CF3SO2)2N]−.
With the proper selection of its ionic components, the ILs proved to be better plasticizers than glycerol. For example, when [C4mim]Cl IL was used as a plasticizer for starch processing, the obtained products usually exhibited lower water absorption [75], with four times from 100 to 400% improved elasticity [76]. Bendaoud et al. have shown that [C4mim]Cl IL destroyed the biopolymeric crystal structure more efficiently when compared to diethylphthalate [73]. This same IL allows a faster de-structurization of the biopolymer during their processing, including starch, which reduces the energy required in this step and the formation of more homogeneous blends [70,71]. Similar results were observed when [Amim]Cl was used as a plasticizer for starch processing: lower glass transition temperatures (Tg) for processing the biopolymeric materials and the resulting materials presented lower water absorption [77,78]. Wang et al. proposed the synthesis of an imidazolium-based polymeric IL (PIL) and used it as a plasticizer for starch. The resulting material was hot-pressed into a transparent film with improved elastic properties [65]. In addition, ILs exhibit high electrical conductivity and wide electrochemical windows and can impart ionic conductivity into biopolymeric architectures to produce biopolymer-based ion-conducting films and fibers.
The IL [C2mim][OAc] is known to significantly destroy the crystalline structure of the biopolymers, increasing the amorphous fraction by replacing inter- and intra-biopolymeric interactions with [C2mim][OAc]-biopolymer ones. Hence, when plasticized with this IL, biopolymers such as starch are suitable for the preparation of starch-based films [79,80,81], and the obtained materials exhibit lower tensile strength and stiffness, but higher flexibility compared to those prepared without the use of IL. Moreover, these films show better anti-aging effects and greater biological stability than films obtained using glycerol as a plasticizer. Zhang et al. demonstrated that [C2mim][OAc] successfully modifies starch into optically transparent electroconductive films [82]. The addition of this IL significantly reduces the processing temperature (used in compression molding) to a relatively mild temperature (ranging between 55 and 65 °C), much lower than those commonly used in biopolymer processing (mostly over 150 °C).
Although imidazolium-based ILs have been demonstrated to be efficient plasticizers for biopolymers, an exploration of the role of the anions has been limited. Romano et al. prepared transparent starch films plasticized with [C2mim]+ combined with different anions, including [SO3Et]−, [N(CN)2]−, [OAc]−, and [Cl]− [79]. In addition, the authors explored the effect of the dicationic IL 3,3′-methylenebis(1-methyl-1H-imidazol-3-ium) dichloride as plasticizer. A decrease in dry Tg values compared with that of native starch was observed for all films. Stress−strain experiments showed that the addition of the IL brought about a substantial increase in the toughness of the otherwise very brittle starch. However, the different anions and the replacement of the monocationic structure with a dicationic structure led to the production of films with different characteristics. Mechanical testing identified the [C2mim]Cl-based film as the most compliant. On the other hand, by replacing the monocationic structure with a dicationic one, the plasticizing effect was reduced [79].
Deep eutectic solvents (DESs) have also been successfully employed as plasticizers for polysaccharide-based films [83,84,85,86,87,88] due to increasing interest in sustainable methods and a shift to more “natural-based” plasticizers. DESs, unlike ILs, are not pure compounds and were originally defined as a mixture of a quaternary ammonium salt with a hydrogen bond donor (HBD) [89]. This definition has expanded since then, terming them as complexes of Lewis or Brønsted acidic HBDs and basic hydrogen bond acceptors (HBAs). The formation of such HBD:HBA complexes is the foundation of the DES character. The use of DES as plasticizers to facilitate the production of thermoplastic starch has been recently extensively reviewed by Skowrońska and Wilpiszewska [90], whereas Wei et al. [81] specifically reviewed choline chloride ([Cho]Cl)-based DES. DES exhibit superior performance compared to traditional plasticizers in the production of materials from chitosan, starch, and cellulose [81,91]. While the poor plasticity of chitosan films and their low mechanical stability have restricted the potential uses of brittle chitosan films, DES improve their low mechanical stability, enhance flexibility, and reduce their fragility. Still, although the use of the DES choline chloride—malonic acid as a plasticizer can improve the properties of chitosan films, i.e., significantly increasing their elasticity and lowering their Young’s modulus in comparison to native chitosan films, the use of highly hydrophilic plasticizers results in a high water vapor transmission rate, and is not recommended for applications such as food-packaging materials [92].
The proper selection of the plasticizer was demonstrated to allow the interactions between non-miscible biopolymers, such as starch and zein particles, to be tuned, influencing the microstructural arrangement between phases [93]. Based on the plasticizer, two distinctive behaviors of mechanical response were described by the authors: elastoplastic (choline acetate, glycerol) and hyperelastic ([C4mim]Cl, glycerol-[Cho]Cl, urea-[Cho]Cl). On the other hand, the introduction of solid particles, such as graphene oxide (GO), reduced graphene oxide (rGO), or clays, can be used to modify the interactions between the ILs and biopolymers, and hence modify the properties of the resulting materials. For example, when GO and rGO were added to mixtures of IL [C2mim][OAc], chitosan, and carboxymethyl cellulose (CMC) and to mixtures of IL [C2mim][OAc], chitosan, and alginate [67,70], the IL played a dominant role as a plasticizer, increasing the mobility of biopolymer chains as well as ions and associated dipoles but reducing biopolymer chain interactions, crystallinity, and thermal stability. The addition of GO strengthened the interactions between the components (GO, biopolymers, and the IL), resulting in enhanced mechanical properties and decreased surface hydrophilicity. This same effect was observed with the addition of clay montmorillonite (MMT) to the chitosan/CMC system and [C2mim][OAc] as a plasticizer: MMT was reported to interrupt the interactions between the IL and the biopolymers, leading to increased surface wettability [94].
This entry is adapted from the peer-reviewed paper 10.3390/ijms25031720
This entry is offline, you can click here to edit this entry!
