Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Lipids are a crucial component of the human brain, serving important structural and functional roles. They are involved in cell function, myelination of neuronal projections, neurotransmission, neural plasticity, energy metabolism, and neuroinflammation. Despite their significance, the role of lipids in the development of mental disorders has not been well understood. This review focused on the potential use of lipids as blood biomarkers for common mental illnesses, such as major depressive disorder, anxiety disorders, bipolar disorder, and schizophrenia.
- lipids
- MDD
- schizophrenia
- bipolar disorder
- anxiety disorder
1. Introduction
The most common mental illnesses are affective disorders and schizophrenia. Thus, among them are major depressive disorder (MDD), anxiety disorders (ADs), bipolar disorder (BPD), and schizophrenia (SCZ) [1]. These illnesses result in long-term disability and cause invalidity. Their disease courses have been characterized by emotional and cognitive disturbances, mood disorders, impaired functioning, and social isolation [1]. In recent years, advances in technology have allowed for the identification of many biological markers of mental illnesses, such as genomic, epigenomic, metabolic, and proteomic markers. However, much less attention has been paid to the lipid markers.
In 2005, the International Committee on the Classification and Nomenclature of Lipids identified eight classes of lipids; they have been displayed in the LIPID MAPS Structure Database [2]. Two fundamental ‘building blocks’ (ketoacyl groups and isoprene groups) form the basis of the LIPID MAPS classification system. Therefore, lipids are defined as hydrophobic or amphipathic small molecules that can arise, in whole or in part, from two types of condensation: based on the carbanions of ketoacyl thioethers and/or based on the carbocations of isoprene units. This classification system segregates eight categories of lipids: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, polyketides (derived from the condensation of ketoacyl subunits), sterol lipids, and prenol lipids (derived from the condensation of isoprene subunits). The classification of these lipids is shown in Figure 1.
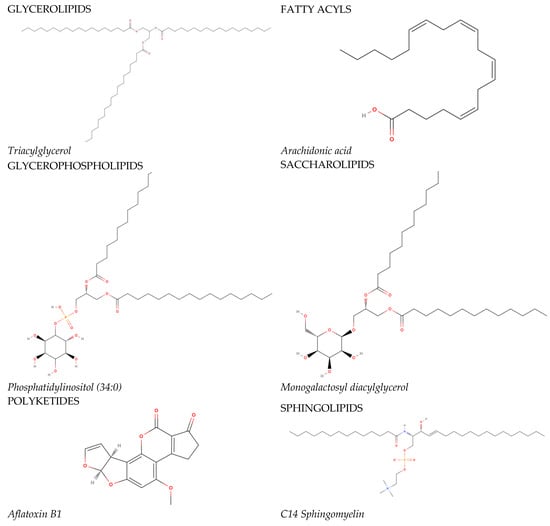
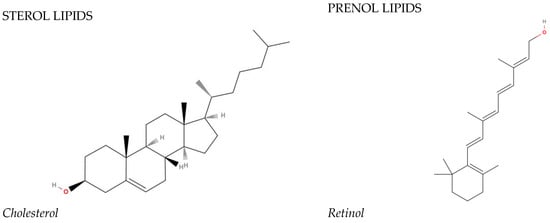
Figure 1. The main lipid groups and the examples of their representatives. The illustrations were prepared using the following program: https://molview.org/ accessed on 18 December 2023).
The brain is the organ that is enriched in lipids. Only adipose tissue contains a larger amount of lipids compared to brain tissue [3]. The entire variety of brain lipids is involved in a range of essential processes, the disruption of which can cause significant damage to the central nervous system (CNS).
Lipids are the structural components of cell membranes, which are involved in a set of processes in the cell, such as myelination, neurotransmission, synaptic plasticity, energy, metabolic processes, and inflammatory processes. Interventions in these processes may influence the development of psychiatric disorders and contribute to their pathogenesis [4].
The state of the cell membrane is extremely important for the functions of neurons and glial cells. In nerve cells, lipids comprise 50–60% of cell membrane components [5]. Lipids form a phospholipid bilayer, the basic structural unit of the membrane, which participates in the regulation of permeability. The three major classes of membrane lipids are glycerophospholipids (e.g., phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE), and phosphatidylinositol (PI)), cholesterol, and sphingolipids. The outer layer of the plasma membrane mainly consists of PC and sphingomyelin (SM); PE and PS represent the predominant phospholipids of the inner layer. PI is also localized in the inner part of the membrane and plays an important role in cell signaling [5]. Sphingolipids (SPs) contain long-chain fatty acids, which provide inter-lipid associations in the lipid bilayer [6]. Cholesterol acts as a “strengthening link” in the structures of membranes, providing them with the necessary strength and stability. Cholesterol affects membrane fluidity and increases the level of friction between membrane flaps [7]. The relative size and degree of fatty acid saturation in lipids affect membrane curvature, fluidity, and thickness [8]. Moreover, lipids can modulate the activity of membrane proteins with lipid-binding domains by recruiting them to specific membrane compartments or subdomains [9]. Lipids also participate in intracellular signaling, where they act as secondary messengers. The most common are diacylglycerol (DG) and inositol triphosphate (IP3) [10].
Myelination plays a crucial role in signal transduction and the proper functioning of the CNS. The main function of myelin is to provide electrical insulation of axons for sufficiently efficient transmission of action potentials. Myelin is characterized by an extremely high lipid content (~80% of dry weight) and a peculiar lipid composition in which the ratio of cholesterol to phospholipids (mainly ethanolamine phosphatide and phosphatidylcholine) to glycolipids (e.g., galactosylceramide and sulfatide) is approximately 2:2:1. Specific SPs and glycerides are substances that cover nerve fibers and accelerate the transmission of nerve impulses [11]. Myelin is particularly high in saturated and monounsaturated lower and higher fatty acids. Phospholipids containing such fatty acids may contribute to the electrical insulation of axons by reducing their membrane fluidity [12].
The functioning and activity of cellular receptors depend on the interactions between the proteins and lipids that comprise the bilipid layer of the cell membrane [13]. Firstly, lipids are involved in the regulation of synapse development and plasticity. For example, levels of tropomyosin receptor kinase B, a crucial protein in synapse development, are regulated via cholesterol levels [14]. Secondly, lipids participate in the release of presynaptic vesicles [15]. Third, lipids regulate neurotransmitter receptors independently, mostly through direct interactions. For example, cholesterol has been shown to function as a direct allosteric regulator of G protein-coupled receptors [16]. Abnormal phospholipid changes have been reported to disturb the functions of ion channels, neurotransmitters, and cell signaling [17].
Lipids also control neuroplasticity. Glycerophospholipids (GPs) and phosphoinositides are important regulators of dendritic spine plasticity. Lipids also influence dendritic spine plasticity by covalently binding to key synaptic proteins via palmitoylation, which can reversibly modulate protein function [18]. Neutral sphingomyelinases also regulate synaptic potentiation. Previous studies have demonstrated at least two different functions of lipids in plasticity processes: altering the functions of synaptic proteins through the palmitoylation mechanism and linking cytoskeletal regulators to membrane remodeling [19]. SPs modulate structural plasticity and neuronal dynamics through lipid–cytoskeletal interactions [20]. Neuronal activity can induce rapid changes in lipid metabolism. It rapidly modulates GP and SP levels. Several studies have shown the effects of ceramide (Cer) metabolism on neuronal susceptibility to death and plasticity process [21]. Cholesterol-deficient neuronal cells exhibit reduced synaptic transmission and impaired synaptic plasticity [4].
Brain tissue needs a large amount of energy. Neurons in the adult brain mainly depend on glucose as an energy source. However, about 20% of the total energy requirements of the adult brain are provided through the oxidation of fatty acids. It has been considered that fatty acid oxidation occurs almost exclusively in astrocytes, and carnitine and fatty acids can be transported from the blood to the astrocytes [22]. Brain mitochondria are characterized by a number of special features. The lipid-to-protein ratio of phospholipids, or cholesterol, is lower in brain mitochondria compared to other organelles [23]. In particular, mitochondria do not contain SM and glycosphingolipids. The major phospholipids of mitochondrial membranes are PC and PE, mainly located in the inner membrane. PI and PS are almost equally distributed on both membranes [23].
Inflammatory processes have been observed during many mental illnesses [24]. Microglial cells are activated during inflammation and perform phagocytosis to counteract inflammation [25]. Once abnormalities are detected, complex remodeling of the lipid composition of microglial cells occurs, providing inflammatory signaling and effector functions [26]. Microglial cells contain receptors for low-density lipoproteins (LDLs) that regulate inflammatory signaling [27]. Cers also promote inflammation and microglia activation [21]. Several lipids represent the sources of pro-inflammatory cytokines that contribute to pathologic neuroinflammatory processes. For example, under certain conditions, arachidonic acid (AA) can produce pro-inflammatory mediators, such as prostaglandins (PGs) and leukotrienes [28]. Long-chain PUFAs (polyunsaturated fatty acids), representing a source of eicosanoids and docosanoids, play an important role in neuroprotective and anti-inflammatory effects in the CNS [4]. SMs also participate in neuroinflammation through cytokine release, microglia activation, and other immune processes [29].
The role of lipids in physiological processes important for the functioning of the nervous system is summarized in Figure 2.
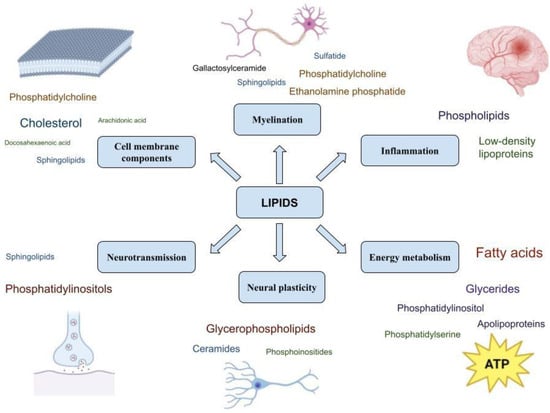
Figure 2. Lipids and their functions in the nervous system.
Therefore, lipids play a major role in the functioning of the nervous system, and alterations in lipid metabolism may influence mental disorder development.
Changes in lipid metabolism in brain tissue may contribute to the pathogenesis of neuropsychiatric diseases. The presence of the blood–brain barrier prevents the free penetration of compounds into the brain. Small lipophilic molecules can pass into brain tissue via passive diffusion; at the same time, all other lipids enter the brain via transcytosis or special transport proteins [23]. For example, unbound long-chain fatty acids can diffuse through the membranes. Meanwhile, cholesterol is almost entirely synthesized in the brain, so its concentration in the blood cannot reflect the processes occurring in the central nervous system. It has been hypothesized that fatty acid metabolism in specific regions of the hypothalamus functions as sensors of nutrient availability that are involved in integrating energy balance through the control of multiple nutritional and hormonal signals. In other brain regions, no differences in glucose and fatty acid metabolism were found, depending on nutritional status [23]. The study of brain lipid composition is an important and urgent task from the point of view of fundamental science. However, for practical purposes, it is necessary to study its associations with blood lipid content.
2. Lipids and Their Role in Neuropsychiatric Disorders
Lipids influence several pathophysiologic pathways that are involved in the development of psychiatric illnesses [30]. The most pronounced effect in the literature has been shown for SCZ and MDD.
Disruption of lipid function is one of the components of SCZ pathogenesis [31]. Yao et al. demonstrated a direct link between abnormal phospholipid levels and disrupted neurochemical parameters, such as SCZ-associated abnormal dopamine and glutamate levels [32]. Phospholipid metabolism abnormalities occur during the progression of SCZ. Most notably, phospholipase A2 (PLA2) activity increases and the level of PUFA integration into phospholipids decreases [31]. The association between PLA2 activity and the dopamine system has also been demonstrated [33]. In particular, it was demonstrated that PUFA dissociation and saturated fatty acid (SFA) incorporation in membrane phospholipids are enhanced in SCZ patients. Decreased levels of membrane phospholipid precursors in the brains of SCZ patients indicate reduced synthesis of PC and PE. Abnormal expression of enzymes and impaired homeostasis of membrane lipids in patients have been associated with the imbalance of phospholipid breakdown and remodeling under the influence of increased oxidative stress. Phospholipid metabolism plays a critical role in the process of synaptic growth, and its dysfunction has been associated with abnormal neuronal development in SCZ [17]. SM and Cers also exert an effect on the presynaptic release of dopamine [34].
In the field of depression research, Andreas Walther et al. proposed their model of lipid involvement in the pathogenesis of depression [35]. This model is based on the chronic stress effects. Chronic stress has been thought to trigger two main pathways: the hypothalamic–pituitary–adrenal axis (HPA) and neuroinflammation [35].
Chronic stress leads to HPA hyperactivity. Elevated glucocorticoid levels increase phospholipase D activity [35]. Increased phospholipase D activity enhances the conversion of PC and PE into phosphatidic acid, as well as lysophosphatidylcholine (LPC) and lysophosphatidylethanolamine (LPE). Due to its chemical properties, phosphatidic acid is rapidly converted into DG. DG, LPC, and LPE cause membrane buckling and destabilization, allowing for a greater glucocorticoid influx into the cell. Together with the above mechanism, elevated glucocorticoid levels decrease triacylglycerol hydrolase expression and enhance triacylglycerol (TAG) biosynthesis by increasing the level of diacylglycerolacyltransferase 2. Decreased triacylglycerol hydrolase expression and increased TAG biosynthesis raise the level of TAG [35]. TAG, in turn, is associated with increased glucocorticoid levels.
Chronic stress also leads to the dysregulation of inflammation. Excess pro-inflammatory cytokines and phasic reagents increase the level of PLA2 [35]. Increased PLA2 activity induces the conversion of PC-containing linoleic acid into AA. AA is subsequently converted into PGs, including pro-inflammatory cytokines (e.g., PGA2, PGD2, PGE2, PGF2, PGH2, and PGI2) [35]. PGs further enhance inflammatory responses.
Increased saturated fatty acid-rich phospholipids, namely lysoPS (16:0), lysoPS (18:0), and SM (24:0), have been associated with inflammation and oxidative stress responses in depressed patients [35]. The elevation of δ-6 desaturase activity in patients with depressive symptoms has been demonstrated. δ-6 desaturase converts linoleic acid into AA, which is a precursor of pro-inflammatory products [36].
Several studies have suggested that omega-3 fatty acid deficiency can decrease dopamine levels, D2 receptor expression and mRNA, presynaptic dopamine vesicle amount, and increase dopamine cleavage [37]. Moreover, its deficiency also downregulates tyrosine hydroxylase activity, which results in reduced dopamine levels and depressive symptomatology development [38].
Increased Cer concentrations may also contribute to the progression of depression, as Cers may affect dopamine transporter function by decreasing dopamine transport and increasing 5HT transport [39]. Moreover, it has been reported that increased Cers may affect monoamine neurotransmitter reuptake and initiate a biological cascade that leads to the downregulation of serotoninergic neurotransmission, which represents another pathophysiologic hallmark of depression [38].
Most of the changes accompanying psychiatric disorders primarily affect brain structures. Lipid metabolism impairments during mental diseases also primarily occur in the CNS tissues. Nevertheless, it is necessary to use the available research methods for diagnostic purposes in clinical practice.
Changes in lipid profiles have been consistently observed in the blood serum and plasma across patients with psychiatric diagnoses.
In particular, a lot of studies have focused on identifying reliable blood lipid indicators in SCZ. In total, over 29 studies have assessed lipid changes in the blood of patients with SCZ compared to healthy controls. Two studies were conducted without a control group. Most of the observed studies included patients that received treatment, but three of them also included patients with first-episode psychosis (FEP). However, only nine of the described studies involved more than 100 individuals.
Nine studies evaluated the broad panels of different lipid markers in patients with SCZ [31,34,40,41,42,43,44,45,46] and detected statistical differences in the following lipid classes: fatty acyls, sterols, glycerolipids, sphingolipids, glycerophospholipids, and products of lipid metabolism. Several studies included information about lipids associated with the membranes of erythrocytes [31,47,48]. The most consistent data from the reviewed studies were obtained for PC, PE, SM, and triacylglycerols (TGs). In particular, reduction in these lipid species was mostly demonstrated. Malondialdehyde—the marker of oxidative stress—was also increased in all the concerned studies. Moreover, a meta-analysis considering malondialdehyde in SCZ was conducted [49]. It was shown that medically treated SCZ patients were more affected by the increased oxidative stress, but malondialdehyde levels were elevated in both the treated and untreated groups, in contrast to other markers of oxidative stress. Bile acids were investigated in two studies, and their levels decreased in both of them [40,50]. Calcifediol was reduced in one observed study [50]. High-density lipoprotein (HDL) was decreased in two studies [51,52]. Studies considering fatty acyl and Cer levels received inconsistent results: some lipid types were decreased and some were increased. The levels of PC and PE did not differ significantly in FEP patients and medicated patients [53,54]. The data addressed to PUFA concentrations were found to be inconsistent.
It is worth highlighting those studies that do not identify differences between healthy individuals and patients with SCZ but rather make associations between blood lipids and symptoms of the illness. In some studies, changes in lipid levels in SCZ patients have been associated with Positive and Negative Syndrome Scale (PANSS) scores. For example, such correlations have been detected for shorter-chain TGs [41] and oxysterols [55]. Nandeesha (2023) [52] showed that total cholesterol (TC) and TG levels were negatively correlated with cognitive scores. Plasma calcifediol levels and the ratio of cholestanol to tchol were found to be negatively correlated with Montreal Cognitive Assessment (MOCA) scores [55]. Baseline membrane linoleic acid levels in SCZ with ultra-high risk (UHR) were associated with conversion to psychosis. Sterol, fatty acid, and phospholipid membrane compositions improved the prediction of the psychosis onset [47]. TC levels were positively associated with the Repeated Battery for the Assessment of Neuropsychological Status (RBANS) subscale scores of immediate memory and language [56]. These results further suggest the potential use of blood lipid profiles for the assessment of SCZ symptomatology.
For MDD, we described nineteen studies, which included one study considering postpartum depression [57] and four studies considering depression symptoms in the healthy population. Four studies above them were conducted on drug-naïve patients. Only four studies on MDD patients included more than one hundred individuals. Six studies evaluated a broad panel of different lipid markers [34,58,59,60,61,62]. Consensual data were received for LPC and LPE measurements, which were increased in the observed studies. PC and malondialdehyde levels were also mostly elevated in the described studies. On the contrary, acylcarnitine (CAR), calcifediol, SM, and bile acids were mostly decreased in the reviewed studies. Inconsistent data were received for PE, PI, Cers, TGs, PUFAs, and SFAs. Two studies on MDD patients and one on postpartum depression individuals reported a reduction in HDL levels. One study indicated an increased level of LDL. Researchers have investigated the levels of cholesteryl ester (CE), TC, sterols, and calcifediol in healthy people with depressive symptoms. Associations with mental symptoms were shown for TC and sterol lipids in women. Several studies have indicated an association between the lipid concentrations of octadecyl-phosphatidylethanolamine (PE-O) [58], SM, and PC-O [63] and symptom severity according to the specific scales.
Fourteen studies were dedicated to the investigation of lipid changes in BPD, including two conducted on drug-naïve patients. Compared to the studies on SCZ and MDD, fewer studies were conducted on patients with BPD. Only one study evaluated the associations of CAR, CE, calcifediol, PE, PC, LPC, LPE, PS, and SM with disease symptoms. Cer and PI levels were increased in two and three studies, respectively. PUFA, TC, and TG changes showed inconsistent associations.
We described 24 studies devoted to the evaluation of blood lipid biomarkers in AD. Eleven studies considered blood lipid constitution during general anxiety disorder (GAD), or AD, including one performed on pregnant women. One study described post-stroke anxiety [64]. Some studies have focused on comorbid psychiatric pathology, such as comorbid AD and MDD or comorbid AD and Parkinson’s disease. The other six works included population studies, which investigated blood lipid biomarkers in individuals with anxiety symptoms. The majority of this research included healthy control or other comparison groups with mental disorders, and only eight did not. Six studies have been conducted on a broad sample of individuals (more than 300). Nevertheless, only a few large metabolomic studies assessing lipid blood constitution in AD were performed [63,65,66]. These broad metabolomic studies have revealed changes in a number of lipids of various classes: fatty acyls, sterol lipids, GP, SP, and glycerolipids [63,65,66].
Most of these studies have focused on investigating the changes in lipoproteins, TGs, and cholesterol. Regarding lipoprotein levels, the results were questionable and multidirectional. Nevertheless, in almost all of the papers that were reviewed, anxiety symptomatology was accompanied by an increase in TGs [67,68,69,70,71] and, in only one, by a reduction [65]. For cholesterol, the reviewed results were also found to be inconsistent. A number of studies have investigated the change in PUFAs in blood during anxiety states and mainly demonstrated their decrease [65,72,73]. Regarding the SFA elevation, multidirectional results have been shown. One study identified a decreased carnitine (propionylcarnitine) level [66]. Bile acid changes were also shown in one study reflecting anxiety symptomatology in MDD [74]. Alterations in Cers have also been found in comorbid pathologies. In particular, Xing et al. demonstrated a positive association of Cer C 20:0 levels with anxiety symptoms in Parkinson’s disease [75]. Unidirectional changes were detected when studying the levels of 20-oxo-22,23,24,25,26,27-hexanorvitamin D3 and malondialdehyde. Thus, decreased levels of 20-oxo-22,23,24,25,26,26,27-hexanorvitamin D3 accompanied anxiety symptomatology [76,77]. Malondialdehyde, on the contrary, was increased in AD patients [62,77]. No changes were identified for calcifediol [78].
Among the addressed studies, one was dedicated to the transdiagnostic lipid markers between four illnesses: MDD, BPD, AD, and SCZ [79]. The authors tried to indicate these transdiagnostic lipid subtypes. Researchers have suggested that 10 lipids can be used for diagnostics across psychiatric disorders. Along with these lipid types, the marker of oxidative stress, malondialdehyde, was increased in all the mentioned psychiatric disorders according to the observed studies. The levels of CAR and SM were also decreased in all reviewed psychiatric disorders. Thus, a number of lipid biomarkers were altered in these mental illnesses. The unidirectionality of some of these changes may indicate the diagnostic potential of blood lipid estimation.
This entry is adapted from the peer-reviewed paper 10.3390/metabo14020080
This entry is offline, you can click here to edit this entry!
