A growing body of evidence has demonstrated that higher consumption of plant-based foods and the nutrients found in vegetarian and plant-based diets are associated with numerous health benefits, including improved blood pressure, glycemic control, lipid levels, body mass index, and acid–base parameters. Furthermore, there has been increasing recognition that vegetarian and plant-based diets may have potential salutary benefits in preventing the development and progression of chronic kidney disease (CKD). While increasing evidence shows that vegetarian and plant-based diets have nephroprotective effects, there remains some degree of uncertainty about their nutritional adequacy and safety in CKD (with respect to protein-energy wasting, hyperkalemia, etc.).
- nutrition
- vegetarian diet
- plant-based diet
- chronic kidney disease
1. Introduction
|
Diet |
CKD Stage |
Protein |
Carbohydrates |
|---|---|---|---|
|
LPD vegan |
3–4 |
0.7 g/kg/day (100% from grain and legumes) |
From cereals |
|
LPDs vegan |
3–4 Indicated in pregnant women with advanced CKD [9], in people at high risk of malnutrition, or in people who do not tolerate legumes [10] |
0.6 g/kg/day (100% from cereals and legumes) + EAAs/KAs (1 tablet every 10 kg of body weight) |
From cereals |
|
PLADO diet |
3–5 |
0.6 g/kg/day (with >50% plant-based sources) |
From whole cereals |
|
PLAFOND diet |
3–5 Diabetic nephropathy |
0.6 to <0.8 g/kg/day (with >50% plant-based sources) |
From whole cereals |
|
VLPDs |
4–5 |
0.3–0.4 g/kg/day + EAAs/KAs (1 tablet every 5 kg of body weight) |
Especially from low-protein substitutes |
LPD: low-protein diet; LPDs: low-protein diet supplemented; PLADO: Plant-Dominant Low-Protein Diet; PLAFOND: patient-centered plant-focused LPD for the nutritional management of CKD/DM; VLPDs: very-low-protein diet supplemented. EAAs/KAs: essential amino acids/keto acids.
2. Overview of Vegetarian and Plant-Based Diets
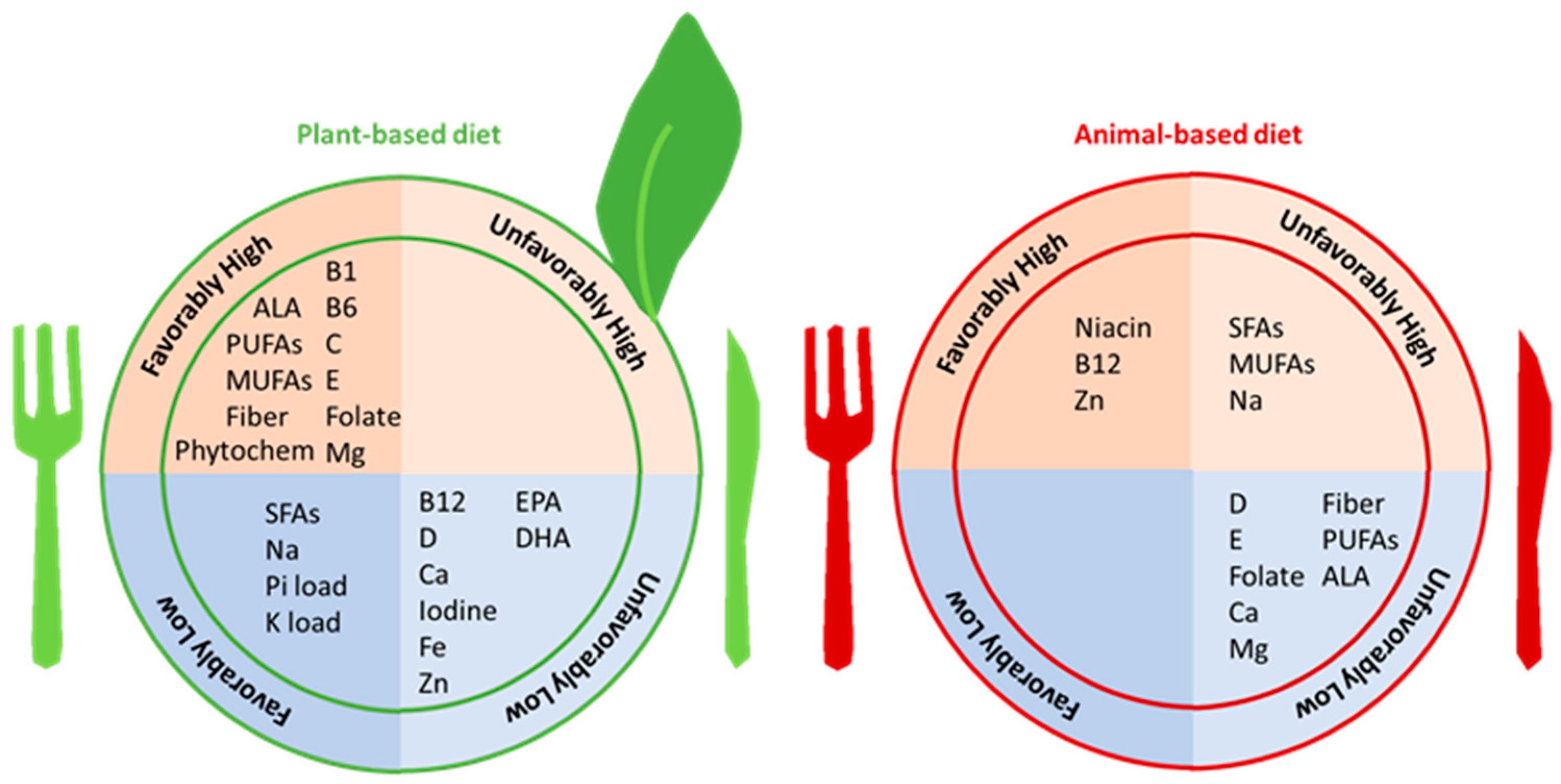
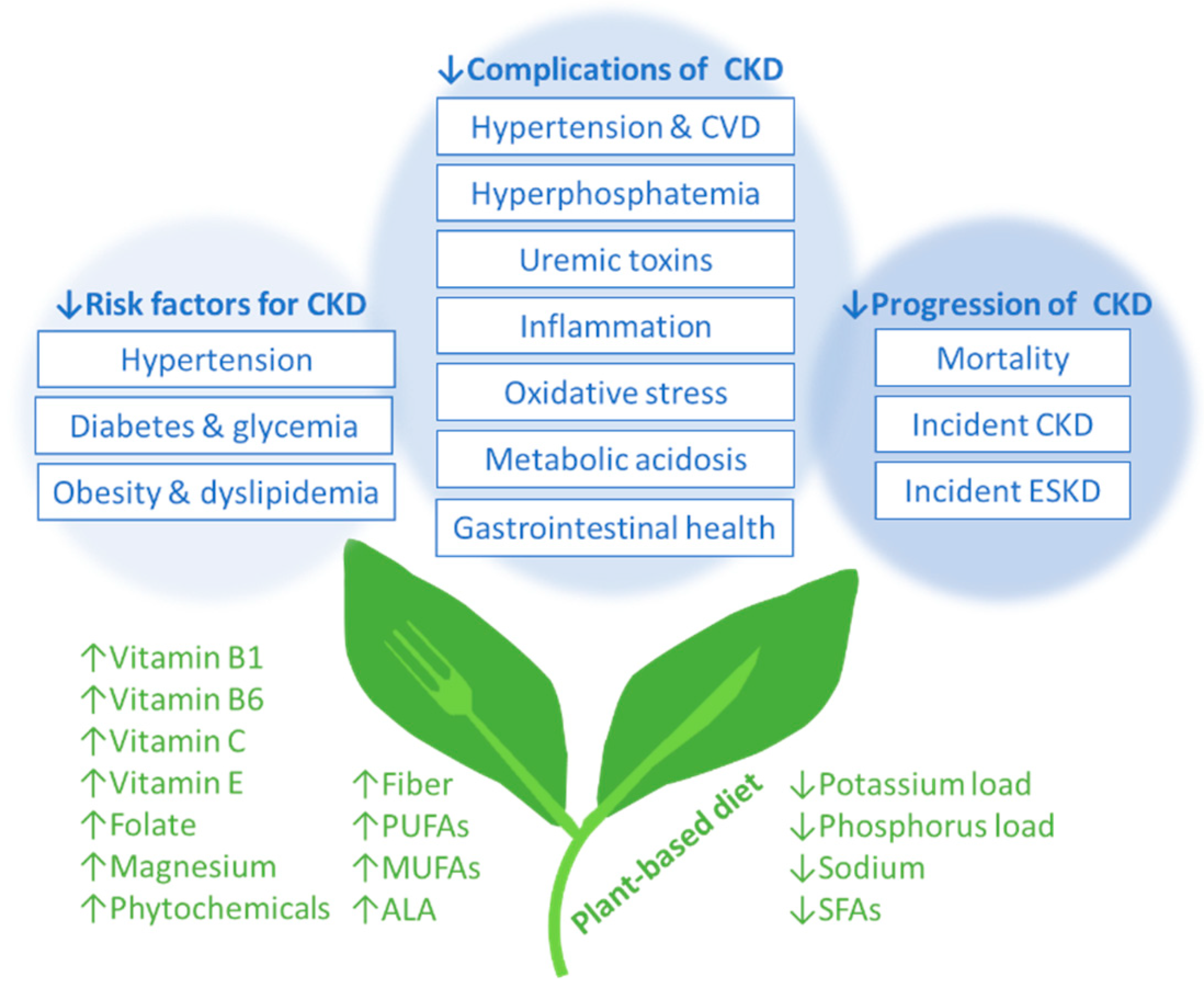
3. Vegetarian Diets and Risk Factors for Incident CKD
3.1. Hypertension in Non-CKD Populations
3.2. Diabetes Mellitus in Non-CKD Populations
4. Vegetarian Diets and CKD Complications
4.1. Hypertension in CKD Populations
|
Property |
Function |
Health Benefits |
|---|---|---|
|
Bulk |
|
|
|
Viscosity |
|
|
|
Fermentability |
|
|
4.2. Hyperphosphatemia in CKD Populations
4.3. Uremic Toxins, Inflammation, and Oxidative Stress in CKD
4.4. Metabolic Acidosis
This entry is adapted from the peer-reviewed paper 10.3390/nu16010066
References
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226.
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039.
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422.
- Baden, M.Y.; Liu, G.; Satija, A.; Li, Y.; Sun, Q.; Fung, T.T.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Bhupathiraju, S.N. Changes in Plant-Based Diet Quality and Total and Cause-Specific Mortality. Circulation 2019, 140, 979–991.
- Bellizzi, V.; Cupisti, A.; Locatelli, F.; Bolasco, P.; Brunori, G.; Cancarini, G.; Caria, S.; De Nicola, L.; Di Iorio, B.R.; Di Micco, L.; et al. Low-protein diets for chronic kidney disease patients: The Italian experience. BMC Nephrol. 2016, 17, 77.
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176.
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931.
- Kalantar-Zadeh, K.; Rhee, C.M.; Joshi, S.; Brown-Tortorici, A.; Kramer, H.M. Medical nutrition therapy using plant-focused low-protein meal plans for management of chronic kidney disease in diabetes. Curr. Opin. Nephrol. Hypertens. 2022, 31, 26–35.
- Reyes-López, M.A.; Piccoli, G.B.; Leone, F.; Orozco-Guillén, A.; Perichart-Perera, O. Nutrition care for chronic kidney disease during pregnancy: An updated review. Eur. J. Clin. Nutr. 2020, 74, 983–990.
- Piccoli, G.B.; Vigotti, F.N.; Leone, F.; Capizzi, I.; Daidola, G.; Cabiddu, G.; Avagnina, P. Low-protein diets in CKD: How can we achieve them? A narrative, pragmatic review. Clin. Kidney J. 2015, 8, 61–70.
- Gluba-Brzózka, A.; Franczyk, B.; Rysz, J. Vegetarian Diet in Chronic Kidney Disease-A Friend or Foe. Nutrients 2017, 9, 374.
- Foundation, N.K. Plant-Based Diet or Vegetarian Diet—What is the Difference? Available online: https://www.kidney.org/atoz/content/plant-based-diet-or-vegetarian-diet-difference (accessed on 13 December 2023).
- Ku, E.; Lee, B.J.; Wei, J.; Weir, M.R. Hypertension in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 74, 120–131.
- Rouse, I.L.; Beilin, L.J.; Armstrong, B.K.; Vandongen, R. Blood-pressure-lowering effect of a vegetarian diet: Controlled trial in normotensive subjects. Lancet 1983, 1, 5–10.
- Margetts, B.M.; Beilin, L.J.; Vandongen, R.; Armstrong, B.K. Vegetarian diet in mild hypertension: A randomised controlled trial. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 1468–1471.
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124.
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern. Med. 2014, 174, 577–587.
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009, 32, 791–796.
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344.
- Kahleova, H.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. A Plant-Based Dietary Intervention Improves Beta-Cell Function and Insulin Resistance in Overweight Adults: A 16-Week Randomized Clinical Trial. Nutrients 2018, 10, 189.
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. A J. Br. Diabet. Assoc. 2011, 28, 549–559.
- Huang, R.Y.; Huang, C.C.; Hu, F.B.; Chavarro, J.E. Vegetarian Diets and Weight Reduction: A Meta-Analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2016, 31, 109–116.
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404.
- Smith, J.D.; Hou, T.; Ludwig, D.S.; Rimm, E.B.; Willett, W.; Hu, F.B.; Mozaffarian, D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: Results from 3 prospective cohorts. Am. J. Clin. Nutr. 2015, 101, 1216–1224.
- Baden, M.Y.; Satija, A.; Hu, F.B.; Huang, T. Change in Plant-Based Diet Quality Is Associated with Changes in Plasma Adiposity-Associated Biomarker Concentrations in Women. J. Nutr. 2019, 149, 676–686.
- Eichelmann, F.; Schwingshackl, L.; Fedirko, V.; Aleksandrova, K. Effect of plant-based diets on obesity-related inflammatory profiles: A systematic review and meta-analysis of intervention trials. Obes. Rev. 2016, 17, 1067–1079.
- Bowman, S.A. A Vegetarian-Style Dietary Pattern Is Associated with Lower Energy, Saturated Fat, and Sodium Intakes; and Higher Whole Grains, Legumes, Nuts, and Soy Intakes by Adults: National Health and Nutrition Examination Surveys 2013-2016. Nutrients 2020, 12, 2668.
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D.; Mudge, D.W.; Kelly, J.T. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev. 2021, 6, Cd010070.
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; De Nicola, L.; Conte, G. Dietary Salt Restriction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2018, 10, 732.
- Shi, H.; Su, X.; Li, C.; Guo, W.; Wang, L. Effect of a low-salt diet on chronic kidney disease outcomes: A systematic review and meta-analysis. BMJ Open 2022, 12, e050843.
- Wang, W.; Soltero, L.; Zhang, P.; Huang, X.R.; Lan, H.Y.; Adrogue, H.J. Renal inflammation is modulated by potassium in chronic kidney disease: Possible role of Smad7. Am. J. Physiol. Ren. Physiol. 2007, 293, F1123–F1130.
- Turban, S.; Juraschek, S.P.; Miller, E.R., 3rd; Anderson, C.A.M.; White, K.; Charleston, J.; Appel, L.J. Randomized Trial on the Effects of Dietary Potassium on Blood Pressure and Serum Potassium Levels in Adults with Chronic Kidney Disease. Nutrients 2021, 13, 2678.
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107.
- Sánchez-Muniz, F.J. Dietary fibre and cardiovascular health. Nutr. Hosp. 2012, 27, 31–45.
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883.
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671.
- Montemurno, E.; Cosola, C.; Dalfino, G.; Daidone, G.; De Angelis, M.; Gobbetti, M.; Gesualdo, L. What would you like to eat, Mr CKD Microbiota? A Mediterranean Diet, please! Kidney Blood Press. Res. 2014, 39, 114–123.
- Altorf-van der Kuil, W.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Bakker, S.J.; Navis, G.; van’t Veer, P.; Geleijnse, J.M. Dietary protein and blood pressure: A systematic review. PLoS ONE 2010, 5, e12102.
- Tielemans, S.M.; Altorf-van der Kuil, W.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Bakker, S.J.; Geleijnse, J.M. Intake of total protein, plant protein and animal protein in relation to blood pressure: A meta-analysis of observational and intervention studies. J. Hum. Hypertens. 2013, 27, 564–571.
- Montalcini, T.; De Bonis, D.; Ferro, Y.; Carè, I.; Mazza, E.; Accattato, F.; Greco, M.; Foti, D.; Romeo, S.; Gulletta, E.; et al. High Vegetable Fats Intake Is Associated with High Resting Energy Expenditure in Vegetarians. Nutrients 2015, 7, 5933–5947.
- Narasaki, Y.; Rhee, C.M. Dietary Therapy for Managing Hyperphosphatemia. Clin. J. Am. Soc. Nephrol. 2020, 16, 9–11.
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530.
- Narasaki, Y.; Yamasaki, M.; Matsuura, S.; Morinishi, M.; Nakagawa, T.; Matsuno, M.; Katsumoto, M.; Nii, S.; Fushitani, Y.; Sugihara, K.; et al. Phosphatemic Index Is a Novel Evaluation Tool for Dietary Phosphorus Load: A Whole-Foods Approach. J. Ren. Nutr. 2020, 30, 493–502.
- Moe, S.M.; Chen, N.X.; Seifert, M.F.; Sinders, R.M.; Duan, D.; Chen, X.; Liang, Y.; Radcliff, J.S.; White, K.E.; Gattone, V.H., 2nd. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009, 75, 176–184.
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A.; Radcliffe, J.S.; Trevino, L.L.; Donahue, S.E.; Asplin, J.R. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 257–264.
- Azadbakht, L.; Esmaillzadeh, A. Soy-protein consumption and kidney-related biomarkers among type 2 diabetics: A crossover, randomized clinical trial. J. Ren. Nutr. 2009, 19, 479–486.
- Cases, A.; Cigarrán-Guldrís, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider. Nutrients 2019, 11, 1263.
- Koppe, L.; Fouque, D.; Soulage, C.O. The Role of Gut Microbiota and Diet on Uremic Retention Solutes Production in the Context of Chronic Kidney Disease. Toxins 2018, 10, 155.
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013, 35, 196–201.
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610.
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306.
- Vaziri, N.D.; Liu, S.M.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Farzaneh, S.H.; Kieffer, D.A.; Adams, S.H.; Martin, R.J. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 2014, 9, e114881.
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870.
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266.
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444.
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863.
- de Brito-Ashurst, I.; Varagunam, M.; Raftery, M.J.; Yaqoob, M.M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J. Am. Soc. Nephrol. 2009, 20, 2075–2084.
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381.
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038.
