Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The discovery of antinuclear antibodies (ANAs) in the mid-20th century during studies on systemic lupus erythematosus (SLE) marked a significant breakthrough.
- antinuclear antibodies
- autoimmune rheumatic diseases
- cancer associations
1. Introduction
The identification of a luminous staining pattern associated with ANAs in 1957 led to further investigations in the 1970s and 1980s, confirming their connection to various autoimmune disorders, expanding beyond SLE to include conditions such as rheumatoid arthritis and systemic sclerosis [1][2]. ANA testing, utilizing methodologies like ELISA and immunoblotting, has since become an indispensable diagnostic tool in the field, providing valuable insights into the diverse patterns that emerge (Figure 1) [1][2][3].
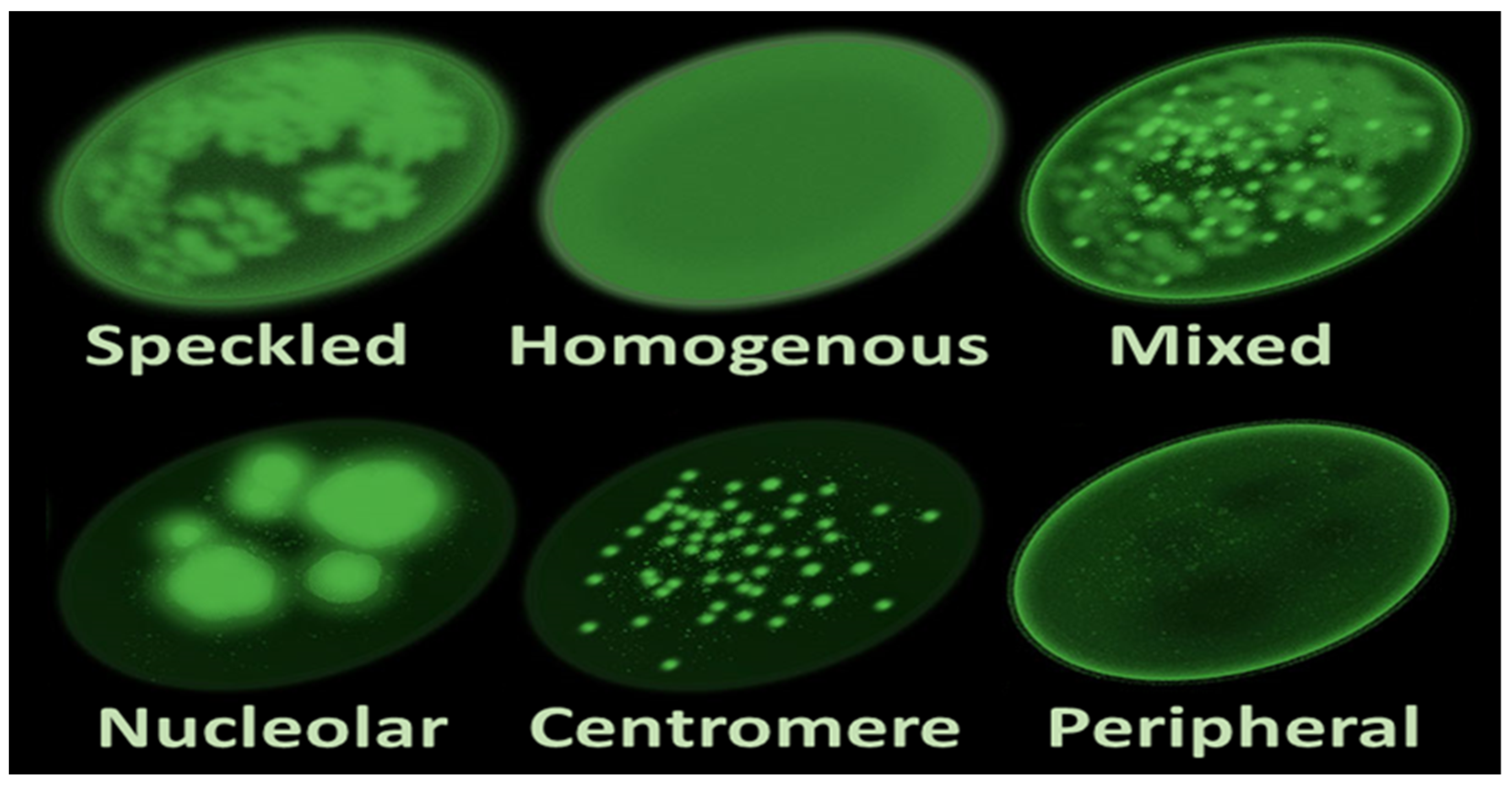
Figure 1. Main antinuclear antibody patterns on immunofluorescence.
Integral to immunological investigations, ANAs offer profound insights into the intricate diagnostic landscape of autoimmune disorders, specifically targeting components within the cell nucleus. Detected through sophisticated laboratory techniques like indirect immunofluorescence (IIF) or enzyme-linked immunosorbent assay (ELISA) [3], ANAs play a crucial role in enhancing the accuracy of diagnostic procedures. Despite their association with autoimmune disorders, it is essential to recognize that not all individuals with positive ANA results definitively have these conditions. Positive ANA results, when considered alongside clinical symptoms and other tests, contribute to a comprehensive diagnostic process [1][2].
2. Clinical Significance of ANAs: Decoding the Diagnostic Symphony
At the nexus of autoimmune rheumatic diseases (ARDs), antinuclear antibodies (ANAs) emerge as crucial sentinels, orchestrating a diagnostic symphony that unfolds within the realms of immunological intricacy. These antibodies, with their propensity to target nuclear and cytoplasmic components, unfurl distinctive fluorescence patterns that serve as invaluable clues in the diagnostic odyssey. While their presence is often synonymous with systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), and scleroderma, decoding ANA patterns unlocks a nuanced understanding of associated autoimmune diseases [2][4][5].
The diagnostic landscape is enriched by the variety of patterns ANAs present under the microscopic lens. Homogeneous, speckled, centromeric, and nucleolar patterns stand as the building blocks of this diagnostic language. A homogenous pattern, often indicative of antibodies against double-stranded DNA (dsDNA), is a hallmark of SLE. Speckled patterns, on the other hand, may suggest antibodies against extractable nuclear antigens (ENAs) and are frequently associated with a spectrum of autoimmune conditions [5].
Centromere patterns, portraying a distinctive speckling at the centromeres of chromosomes, are strongly linked with limited cutaneous systemic sclerosis (lcSSc) and CREST syndrome (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia). Nucleolar patterns, characterized by a bright staining of the nucleoli, are often associated with scleroderma and systemic sclerosis [6].
However, the diagnostic narrative extends beyond these patterns. The clinical significance of ANAs resides not only in their presence, but in the specific patterns they weave. Understanding the intricate dance of these antibodies is akin to deciphering a diagnostic code that directs clinicians toward accurate disease classification [4].
While ANAs are considered hallmark autoantibodies, their diagnostic specificity can be challenging. A positive ANA result prompts the need for further investigations to unveil the specificities within this diagnostic umbrella. Clinicians, armed with a discerning eye, delve into the patient’s clinical history, symptoms, and specialized laboratory tests to differentiate between autoimmune and non-autoimmune conditions [7].
The diagnostic prowess of ANAs extends beyond disease classification. These antibodies, acting as beacons in the diagnostic landscape, provide valuable information about disease prognosis, potential complications, and treatment responses. In conditions like SLE, where ANAs are almost omnipresent, the specific patterns and titers aid in delineating the disease severity and guiding therapeutic strategies [8]. The clinical significance of ANAs becomes even more pronounced in the realm of autoimmune liver diseases. Primary biliary cholangitis (PBC) and autoimmune hepatitis (AIH) often exhibit distinctive ANA patterns, contributing to accurate disease identification and subsequent management. The nuanced understanding of these patterns plays a pivotal role in guiding therapeutic decisions and predicting disease progression [9][10].
In conclusion, the clinical significance of ANAs extends beyond their status as diagnostic markers. They embody a diagnostic symphony, each pattern and specificity composing a unique melody that guides clinicians through the intricate landscape of autoimmune diseases. Decoding this symphony requires not only technical precision in laboratory testing but a nuanced understanding of the clinical context. As research continues to unveil the intricacies of ANAs, their role in autoimmune disease diagnostics promises to evolve, offering clinicians more refined tools for accurate disease identification and personalized therapeutic interventions.
3. Differential Diagnosis in Positive ANA Patients: Navigating Complexity
A positive antinuclear antibody (ANA) test result is a diagnostic starting point that requires careful consideration of various factors to elucidate the underlying health condition. The presence of ANAs in an individual’s serum indicates an immune system response targeting the body’s own cell nuclei, often associated with autoimmune diseases.
3.1. Connective Tissue Diseases (CTDs)
Positive ANA findings are strongly associated with a spectrum of connective tissue diseases, serving as a hallmark for conditions like systemic lupus erythematosus (SLE), systemic sclerosis (SSc), rheumatoid arthritis (RA), and Sjögren’s syndrome. The distinct ANA patterns observed through immunofluorescence on HEp-2 cells aid in narrowing down the specific CTD. For instance, a speckled pattern is commonly linked to SLE, while a centromere pattern is indicative of limited cutaneous SSc [11][12][13].
3.2. Systemic Lupus Erythematosus (SLE)
SLE, a prototypical autoimmune disease, often manifests with a positive ANA result. However, a positive ANA result alone does not confirm SLE, as these antibodies can also be present in other autoimmune conditions. Diagnostic criteria, including clinical manifestations and additional specific autoantibodies such as anti-double-stranded DNA (anti-dsDNA) and anti-Smith (anti-Sm) antibodies, are crucial for a definitive SLE diagnosis [14][15].
3.3. Rheumatoid Arthritis (RA)
While RA is primarily characterized by the presence of rheumatoid factor and anti-citrullinated protein antibodies (ACPA), some RA patients may exhibit positive ANA results. The coexistence of ANAs in RA can contribute to joint inflammation and complicate the overall clinical picture, necessitating a comprehensive assessment for optimal management [16].
3.4. Sjögren’s Syndrome
Positive ANA findings, particularly with a speckled pattern, are common in Sjögren’s syndrome. However, the distinctive involvement of exocrine glands, leading to symptoms such as dry eyes and dry mouth, is pivotal for confirming the diagnosis. Additional testing for anti-Ro (SSA) and anti-La (SSB) antibodies further supports the identification of Sjögren’s syndrome [17].
3.5. Systemic Sclerosis (SSc)
Systemic sclerosis, or scleroderma, often presents with a positive ANA result, and specific ANA patterns like a nucleolar or centromere pattern can guide towards the limited or diffuse form of the disease. However, clinical manifestations, including skin thickening and internal organ involvement, are vital for accurate classification and management [18][19].
3.6. Drug-Induced Lupus
Certain medications can induce lupus-like symptoms, accompanied by positive ANA results. Distinguishing drug-induced lupus from idiopathic SLE is essential for appropriate intervention. Discontinuation of the implicated medication often leads to the resolution of symptoms and ANA titers [20][21].
3.7. Infections and Other Autoimmune Conditions
Infections, such as viral hepatitis and human immunodeficiency virus (HIV), can evoke positive ANA results. Additionally, other autoimmune conditions like autoimmune hepatitis, inflammatory myopathies, and mixed connective tissue disease (MCTD) should be considered in the differential diagnosis [22].
3.8. Overlap Syndromes
Some individuals may exhibit features of multiple autoimmune diseases, leading to overlap syndromes. These cases require meticulous evaluation to discern the predominant disease and guide appropriate therapeutic strategies [23].
In navigating the complex landscape of positive ANA patients, the differential diagnosis extends beyond autoimmune diseases to encompass a spectrum of conditions with potential ANA positivity. Clinical correlation, incorporation of specific autoantibody profiles, and adherence to established diagnostic criteria are pivotal for accurate disease classification and subsequent management. The nuanced approach to differential diagnosis ensures that individuals with positive ANA results receive tailored and timely interventions, optimizing outcomes in the diverse array of health conditions associated with ANA positivity.
4. ANAs and Cancer: A Complex Interplay
As researchers navigate the expansive terrain of antinuclear antibodies (ANAs), their significance transcends the boundaries of autoimmune diseases, reaching into the intricate realm of cancer. Initially recognized for their association with autoimmune disorders such as systemic lupus erythematosus (SLE) and scleroderma, ANAs have emerged as enigmatic players in the complex landscape of tumorigenesis. The interplay between ANAs and cancer introduces a multifaceted narrative, challenging traditional perceptions and prompting a deeper exploration of their role as potential markers and influencers in oncological contexts [5].
The detection of ANAs traditionally involved immunofluorescent techniques, notably utilizing human epithelial cell lines (HEp-2) as substrates. These techniques unravel distinct staining patterns that act as fingerprints, indicative not only of autoimmune processes but also of potential connections to malignancies. Efforts to standardize classification and nomenclature of these patterns, such as the international consensus on ANA patterns (ICAP), have been pivotal in enhancing the understanding of their implications in various diseases, including cancer [24].
Studies have progressively unveiled the intriguing association between ANAs and cancer, challenging the conventional belief that these antibodies are exclusive to autoimmune contexts [25]. The presence of ANAs preceding cancer diagnoses has been particularly notable, with certain studies showcasing their occurrence in lung and colon cancer patients before the manifestation of overt clinical symptoms. This pre-existence of ANAs raises intriguing questions about their potential as early markers or indicators of certain malignancies, heralding a paradigm shift in their clinical relevance [26].
However, the relationship between ANAs and cancer is far from straightforward. While some studies suggest a correlation between ANAs and improved survival rates in specific cancer types, their presence also poses challenges. ANAs can lead to autoimmune manifestations, complicating clinical diagnoses and introducing complexities in patient management. This dual nature of ANAs in cancer patients underscores the complexity of their roles in both autoimmune conditions and malignancies [27].
Specific ANA staining patterns have come under scrutiny for their potential associations with particular cancers. For instance, antibodies conventionally linked to SLE, such as anti-dsDNA, have surfaced in various cancers, including bronchogenic carcinoma and lung cancer’s malignant pleural effusions. Notably, these antibodies have displayed potential prognostic significance, particularly in predicting thymoma relapse or serving as biomarkers in colorectal cancer [24].
Moreover, the exploration extends to other intricate staining patterns like anti-DFS70, anti-centromere, anti-Ro/SSA, anti-La/SSB, and anti-Sm antibodies, each elucidating their associations with certain diseases and their potential roles as prognostic markers or therapeutic predictors in different malignancies. The evolving understanding of ANAs in cancer holds promising implications for diagnosis, treatment, and prognosis, underscoring the urgent need for comprehensive research to uncover the precise mechanisms and roles of ANAs in cancer pathogenesis [24][25].
This enigmatic intersection between ANAs and cancer introduces a dynamic layer to the comprehension of both autoimmune and oncological processes. The antibodies, traditionally perceived through the lens of autoimmune diseases, emerge as potential biomarkers and influential factors in the context of cancer. The complexity of their roles, intertwined with the intricacies of tumorigenesis, invites further exploration, and underscores the need for tailored approaches to deciphering the dual impact of ANAs in autoimmune conditions and cancer [27].
In unraveling the complexities of ANAs and their association with cancer, research has identified specific antibodies that sporadically correlate with solid tumors. One such example is the presence of anti-RNP antibodies, which in certain case studies have shown correlation with metastatic undifferentiated carcinoma—a rare childhood malignancy previously unassociated with anti-RNP antibodies [28].
Additionally, studies have highlighted a notable link between anti-RNA polymerase III antibodies and an elevated risk of cancer in patients with systemic sclerosis (SSc). These antibodies have been significantly associated with an increased likelihood of concomitant malignancy, leading researchers to recommend regular cancer screening for individuals testing positive for anti-RNA polymerase III antibodies in the context of SSc [29][30].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics14030320
References
- Ling, M.; Murali, M. Antinuclear Antibody Tests. Clin. Lab. Med. 2019, 39, 513–524.
- Al-Mughales, J.A. Anti-Nuclear Antibodies Patterns in Patients with Systemic Lupus Erythematosus and Their Correlation with Other Diagnostic Immunological Parameters. Front. Immunol. 2022, 13, 850759.
- Ge, Q.; Gu, X.; Yu, W.; Zhang, G.; Liang, W.; Li, M.; Zhai, G.; Yan, M. Antinuclear antibodies in healthy population: Positive association with abnormal tissue metabolism, inflammation and immune dysfunction. Int. Immunopharmacol. 2022, 113, 109292.
- Bossuyt, X.; De Langhe, E.; Borghi, M.O.; Meroni, P.L. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2020, 16, 715–726.
- Vlagea, A.; Falagan, S.; Gutiérrez-Gutiérrez, G.; Moreno-Rubio, J.; Merino, M.; Zambrana, F.; Casado, E.; Sereno, M. Antinuclear antibodies and cancer: A literature review. Crit. Rev. Oncol. Hematol. 2018, 127, 42–49.
- Andrade, L.E.C.; Damoiseaux, J.; Vergani, D.; Fritzler, M.J. Antinuclear antibodies (ANA) as a criterion for classification and diagnosis of systemic autoimmune diseases. J. Translat. Autoimmun. 2022, 5, 100145.
- Damoiseaux, J. The International Consensus on ANA Patterns (ICAP): From conception to implementation. Clin. Chem. Lab. Med. 2023, in press.
- Nanda, R.; Gupta, P.; Patel, S.; Shah, S.; Mohapatra, E. Uncommon antinuclear antibody patterns as diagnostic indicators. Clin. Biochem. 2021, 90, 28–33.
- Patsinakidis, N.; Gambichler, T.; Lahner, N.; Moellenhoff, K.; Kreuter, A. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J. Eur. Acad. Dermatol. Venereol. JEADV 2016, 30, 2097–2104.
- Satoh, M.; Ceribelli, A.; Hasegawa, T.; Tanaka, S. Clinical Significance of Antinucleolar Antibodies: Biomarkers for Autoimmune Diseases, Malignancies, and others. Clin. Rev. Allergy Immunol. 2022, 63, 210–239.
- Sapkota, B.; Al Khalili, Y. Mixed Connective Tissue Disease. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023.
- Ferrara, C.A.; La Rocca, G.; Ielo, G.; Libra, A.; Sambataro, G. Towards Early Diagnosis of Mixed Connective Tissue Disease: Updated Perspectives. ImmunoTargets Ther. 2023, 12, 79–89.
- Sharp, G.C.; Irvin, W.S.; Tan, E.M.; Gould, R.G.; Holman, H.R. Mixed connective tissue disease—An apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am. J. Med. 1972, 52, 149–159.
- Lou, H.; Ling, G.S.; Cao, X. Autoantibodies in systemic lupus erythematosus: From immunopathology to therapeutic target. J. Autoimmun. 2022, 132, 102861.
- Jacob, N.; Stohl, W. Autoantibody-dependent and autoantibody-independent roles for B cells in systemic lupus erythematosus: Past, present, and future. Autoimmunity 2010, 43, 84–97.
- Martins, A.; Oliveira, D.; Martins, F.R.; Rato, M.S.; Pinheiro, F.O.; Fonseca, D.; Garcia, S.; Fernandes, B.M.; Pimenta, S.; Vaz, C.; et al. The impact of antinuclear antibodies seroconversion induced by anti-tumor necrosis factor α agents on the clinical outcomes in rheumatic patients. The impact of antinuclear antibodies seroconversion induced by anti-tumor necrosis factor α agents on the clinical outcomes in rheumatic patients. ARP Rheumatol. 2023, in press.
- Arcani, R.; Bertin, D.; Bardin, N.; Mazodier, K.; Jean, R.; Suchon, P.; Venton, G.; Daumas, A.; Jean, E.; Villani, P.; et al. Anti-NuMA antibodies: Clinical associations and significance in patients with primary Sjögren’s syndrome or systemic lupus erythematosus. Rheumatology 2021, 60, 4074–4084.
- Kuwana, M. Circulating Anti-Nuclear Antibodies in Systemic Sclerosis: Utility in Diagnosis and Disease Subsetting. J. Nippon. Med. Sch. 2017, 84, 56–63.
- Cavazzana, I.; Vojinovic, T.; Airo’, P.; Fredi, M.; Ceribelli, A.; Pedretti, E.; Lazzaroni, M.G.; Garrafa, E.; Franceschini, F. Systemic Sclerosis-Specific Antibodies: Novel and Classical Biomarkers. Clin. Rev. Allergy Immunol. 2023, 64, 412–430.
- Pan, X.; Yuan, Y.; Huang, F.; Tian, M. Severe lupus induced by the tumor necrosis factor-alpha inhibitor Anbainuo: A case report. J. Int. Med. Res. 2021, 49, 3000605211022510.
- Vaglio, A.; Grayson, P.C.; Fenaroli, P.; Gianfreda, D.; Boccaletti, V.; Ghiggeri, G.M.; Moroni, G. Drug-induced lupus: Traditional and new concepts. Autoimmun. Rev. 2018, 17, 912–918.
- Grygiel-Górniak, B.; Rogacka, N.; Puszczewicz, M. Antinuclear antibodies in healthy people and non-rheumatic diseases—Diagnostic and clinical implications. Reumatologia 2018, 56, 243–248.
- Lorenzo-Barreto, P.; Roy-Ariño, G.; Pérez-Trapote, F.; Sáez-Marín, A.; Stiauren-Fernández, E.S.; Zarza-Sanz, B.; García Barragán, N.; de la Puente-Bujidos, C.; Buisán-Catevilla, F.J. Rheumatoid meningitis in a patient with overlap syndrome: The usefulness of anti-citrullinated peptide antibodies determination in CSF. Mod. Rheumatol. Case Rep. 2023, 7, 347–349.
- Gauderon, A.; Roux-Lombard, P.; Spoerl, D. Antinuclear Antibodies with a Homogeneous and Speckled Immunofluorescence Pattern Are Associated with Lack of Cancer While Those with a Nucleolar Pattern with the Presence of Cancer. Front. Med. 2020, 7, 165.
- Koga, T.; Okamoto, M.; Satoh, M.; Fujimoto, K.; Zaizen, Y.; Chikasue, T.; Sumi, A.; Kaieda, S.; Matsuo, N.; Matama, G.; et al. Positive Autoantibody Is Associated with Malignancies in Patients with Idiopathic Interstitial Pneumonias. Biomedicines 2022, 10, 2469.
- Yoshifuji, H.; Fujii, T.; Kobayashi, S.; Imura, Y.; Fujita, Y.; Kawabata, D.; Usui, T.; Tanaka, M.; Nagai, S.; Umehara, H.; et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 2006, 39, 233–241.
- Shibata, M.; Makioka, K.; Hamaguchi, Y.; Ikeda, Y. Favourable complete remission of anti-OJ antibody-positive myositis after lung cancer resection. Rheumatology 2022, 61, e77–e79.
- Foster, H.E.; Malleson, P.N.; Petty, R.E.; Cabral, D.A. Anti-RNP antibody in a child with undifferentiated carcinoma and no evidence of mixed connective tissue disease. Br. J. Rheumatol. 1997, 36, 289–291.
- Lepri, G.; Catalano, M.; Bellando-Randone, S.; Pillozzi, S.; Giommoni, E.; Giorgione, R.; Botteri, C.; Matucci-Cerinic, M.; Antonuzzo, L.; Guiducci, S. Systemic Sclerosis Association with Malignancy. Clin. Rev. Allergy Immunol. 2022, 63, 398–416.
- Lopez, L.; Barnetche, T.; Galli, G.; Seneschal, J.; Blanchard, E.; Shipley, E.; Pellegrin, J.L.; Lazaro, E.; Constans, J.; Duffau, P.; et al. Clinical and immunological features of patients with cancer-associated systemic sclerosis: An observational study. Jt. Bone Spine 2023, 90, 105555.
This entry is offline, you can click here to edit this entry!
