Efflux pump inhibitors, small molecules capable of restoring the effectiveness of existing antibiotics, are considered potential solutions to antibiotic resistance and have been an active area of research in recent years. Efflux pump inhibitors block efflux pumps through one or more processes, which can inactivate drug transport.
- efflux pump inhibitor
- drug resistance
- biofilm
- virulence
- bacterial persister cells
1. Introduction
2. Mechanisms of Bacterial Resistance
2.1. The Main Resistance Mechanisms of Common Clinical Pathogens
| Bacteria | Drug Resistance Mechanism | References |
|---|---|---|
| Staphylococcus aureus | Reduced affinity for penicillin-binding proteins and the simultaneous production of β-lactamases to destroy the antibacterial activity of the drug. | [8][9][10] |
| An eEnhanced efflux effect; the most effective multidrug resistance system is NorA. | ||
| A decrease in cell membrane permeability affects the energy metabolism of bacteria, reduces the absorption of drugs, and leads to drug resistance. | ||
| Klebsiella pneumoniae | The generation of 16S rRNA methylesterase and β-lactamase, the mutation of target genes, the generation of multidrug resistance efflux pumps, and the modification of enzymes and target protection proteins. | [11] |
| Pseudomonas aeruginosa | Metallo-β-lactamase-producing enzymes, target mutations, alterations in membrane pore proteins, and active exocytosis systems. | [12] |
| Escherichia coli | The production of β-lactamase, the inhibition of bacterial DNA topoisomerase, and a reduction in drug uptake and increase in efflux through outer membrane alteration. | [13] |
| Campylobacter jejuni | Alteration of antibiotic targets or their expression, exocytosis (e.g., CmeABC), and the modification or inactivation of antibiotics. | [14] |
2.2. Bacterial Efflux System
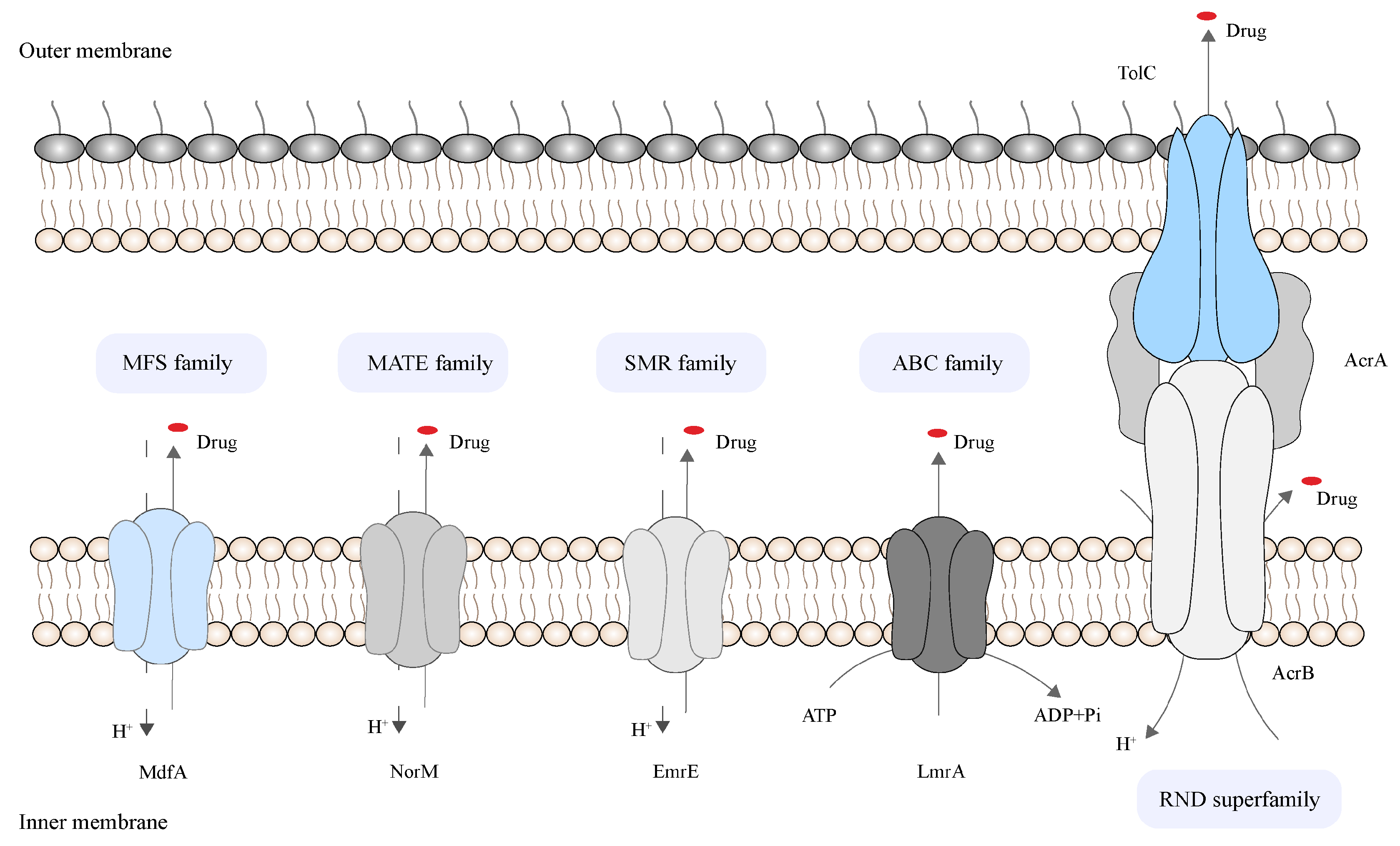
| Bacteria | Efflux Pump | Family | Efflux Substrate | References |
|---|---|---|---|---|
| Staphylococcus aureus | Nor A | MFS | β-lactams, fluoroquinolones, tetracyclines, fungicides, dyes, quaternary ammonium compounds, and preservatives | [22][23][24] |
| Klebsiella pneumoniae | AcrAB-TolC | RND | β-lactams, macrolides, fluoroquinolones, tetracycline, chloramphenicol, cationic dyes, and detergents | [25] |
| OqxAB | RND | Quinolones, chloramphenicol, olaquindox, and tigecycline | ||
| Pseudomonas aeruginosa | MexAB-OprM | RND | Quinolones, tetracyclines, macrolides, chloramphenicol, neomycin, and lincomycin, but not the β-lactam imipenem | [6][26] |
| MexCD-OprJ | Quinolones, tetracyclines, macrolides, neomycin, lincomycin, chloramphenicol, flomoxef, and meropenem | |||
| Escherichia coli | AcrAB-TolC | RND | Fluoroquinolones, tetracyclines, macrolides, rifampin, linezolid, oxazolidinone, novobiocin, clindamycin, and chloramphenicol | [27] |
| Campylobacter jejuni | CmeABC | RND | Macrolides, fluoroquinolones, tetracyclines, rifampin, toxic chemicals, and bile acids | [28][29][30] |
3. The Role of Efflux Pump Inhibitors in Reversing Drug Resistance
3.1. The Status of Drug Resistance Reversal by Efflux Pump Inhibitors
| Source | Inhibitor Type | Representative Drugs | Suppressed Efflux Pumps | References |
|---|---|---|---|---|
| Plants | Alkaloids | Reserpine | NorA | [32] |
| Berberine | MexXY-OprM and NorA | [33] | ||
| Piperine | NorA, MdeA, and Rv1258c | [34][35][36] | ||
| Flavonoids | Astragalusin, kaempferol, silymarin | NorA | [32][37][38] | |
| Chalcone | NorA and MepA | [39] | ||
| Lignocaine | MsrA | [40] | ||
| Phenolic metabolites | 5′-MHC | NorA | [41] | |
| Fermentation products | EA-371α and EA-371δ | MexAB-OprM | [15] | |
| Gallic acid | Epigallocatechin gallate | TetK, MexAB-OprM, and CmeABC | [42][43] | |
| Chemical synthesis | Pyranopyridine | MBX2319 | AcrAB-TolC | [44] |
| Peptides | Phe-Arg-β-naphthylamide | MexAB-OprM, MexEF-OprN, and MexCD-OprJ | [45] | |
| Phenothiazines | Chlorpromazine | AcrB | [46] | |
| Aryl piperazine derivatives | NMP | AcrAB and AcrEF | [47] | |
| Pyridopyrimidine derivatives | D13-9001 | AcrB and MexB | [48] |
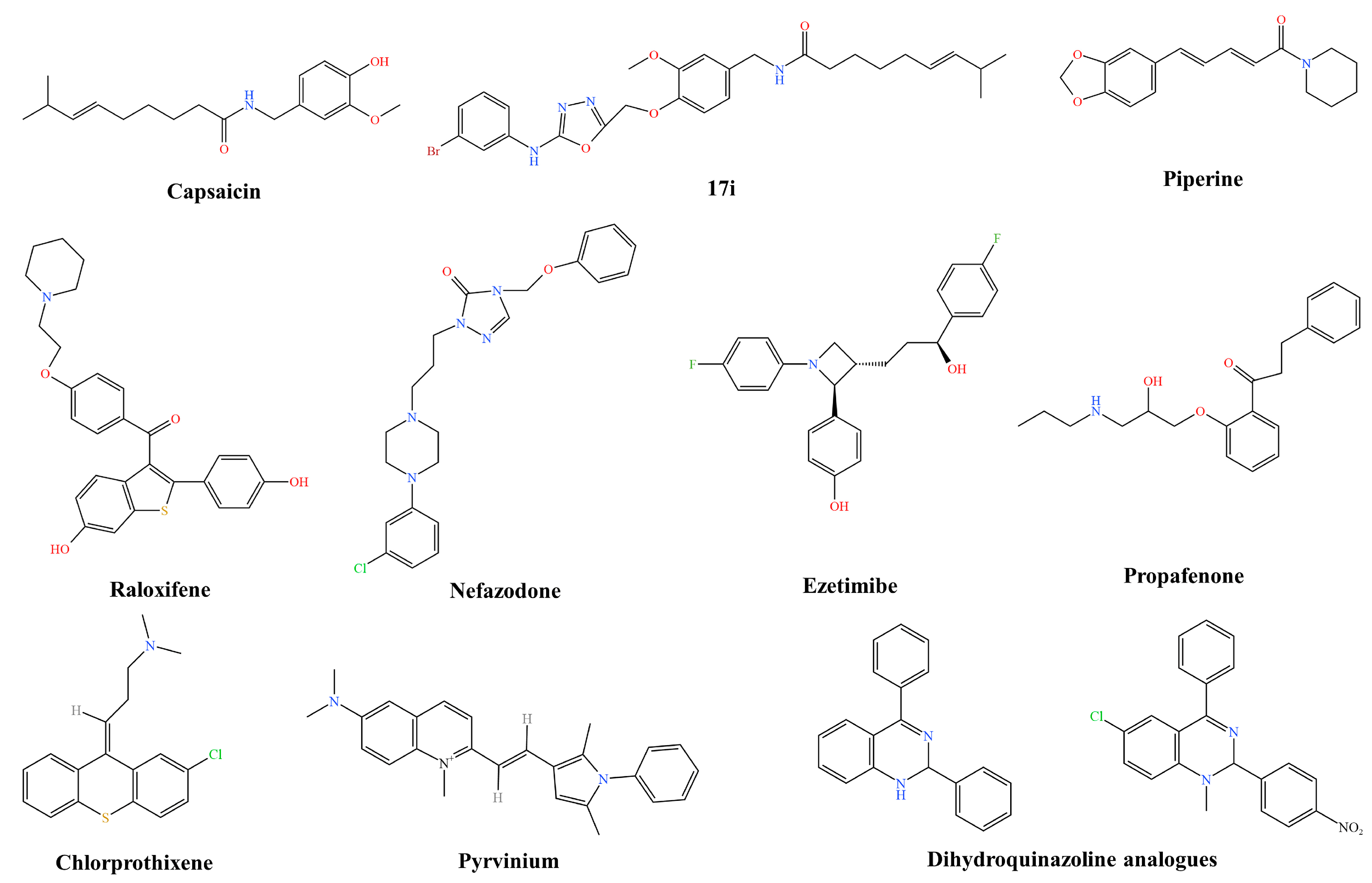
3.1.1. Staphylococcus aureus Efflux Pump Inhibitors
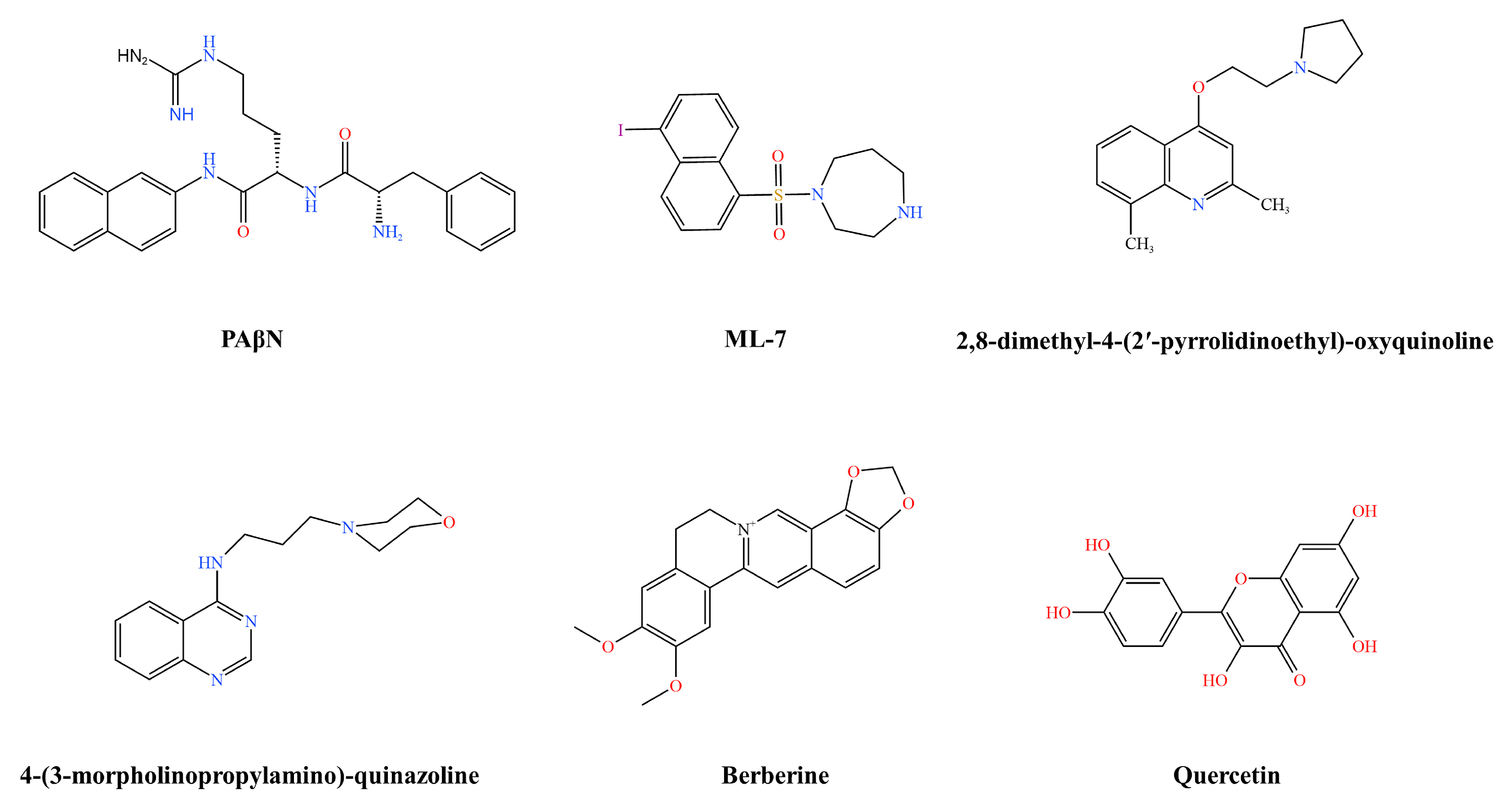
3.1.2. Klebsiella pneumoniae Efflux Pump Inhibitors
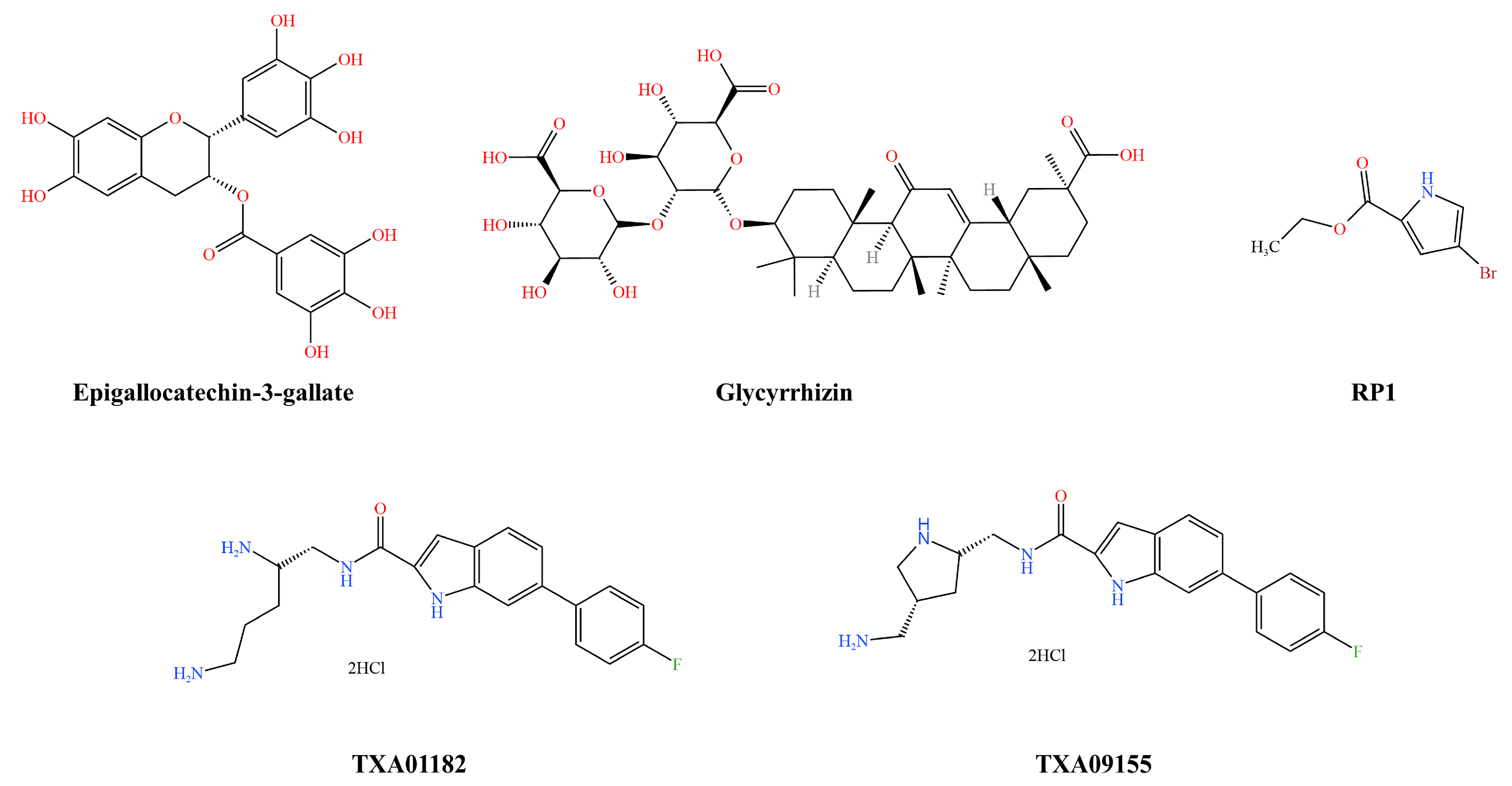
3.1.3. Pseudomonas aeruginosa Efflux Pump Inhibitors
3.1.4. Escherichia coli Efflux Pump Inhibitors
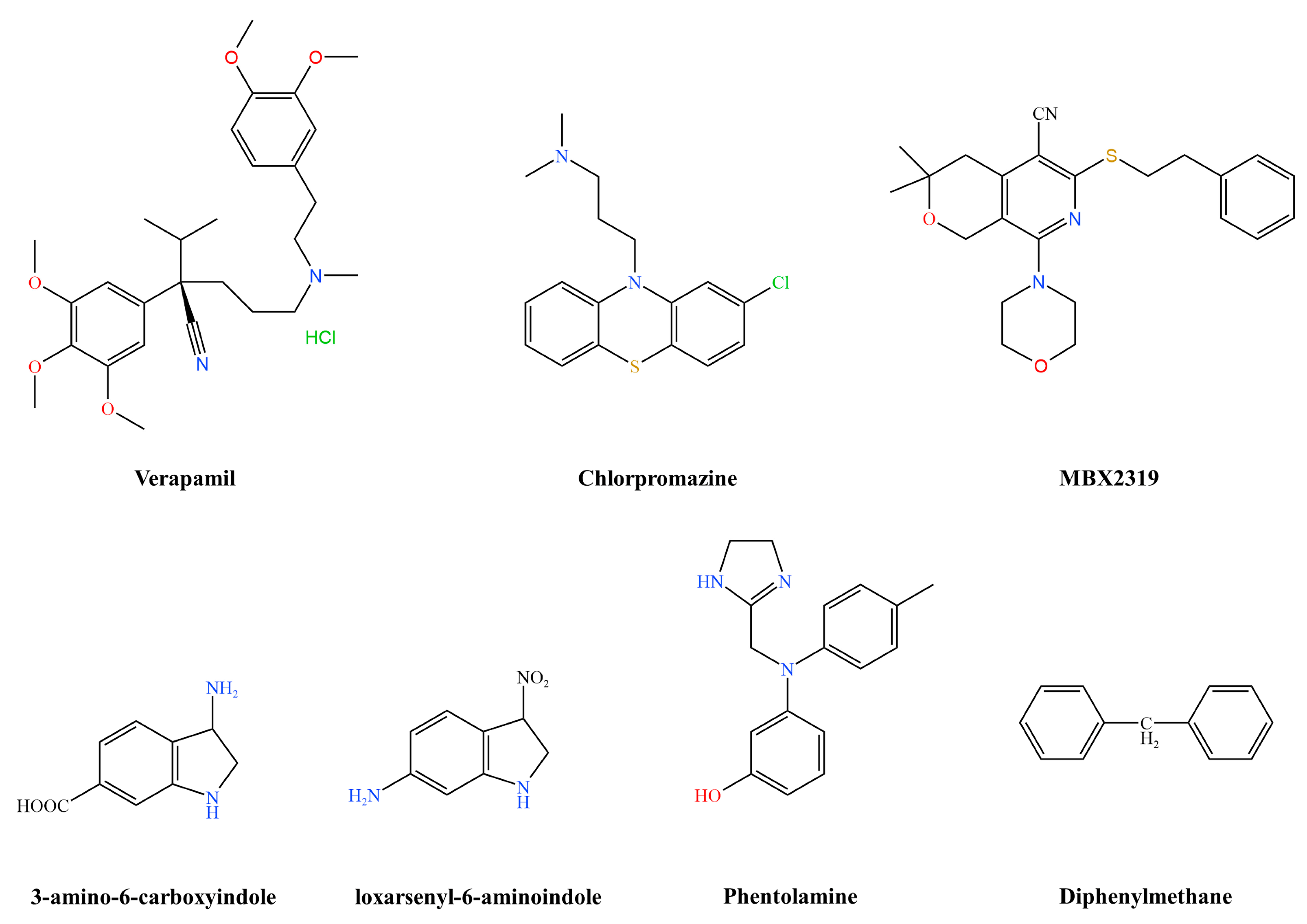
3.1.5. Campylobacter jejuni Efflux Pump Inhibitor
3.2. Screening Methods and Key Technologies of Efflux Pump Inhibitors
4. The Gain Effect of Efflux Pump Inhibitors
4.1. Efflux Pump Inhibitors Inhibit Biofilm Formation
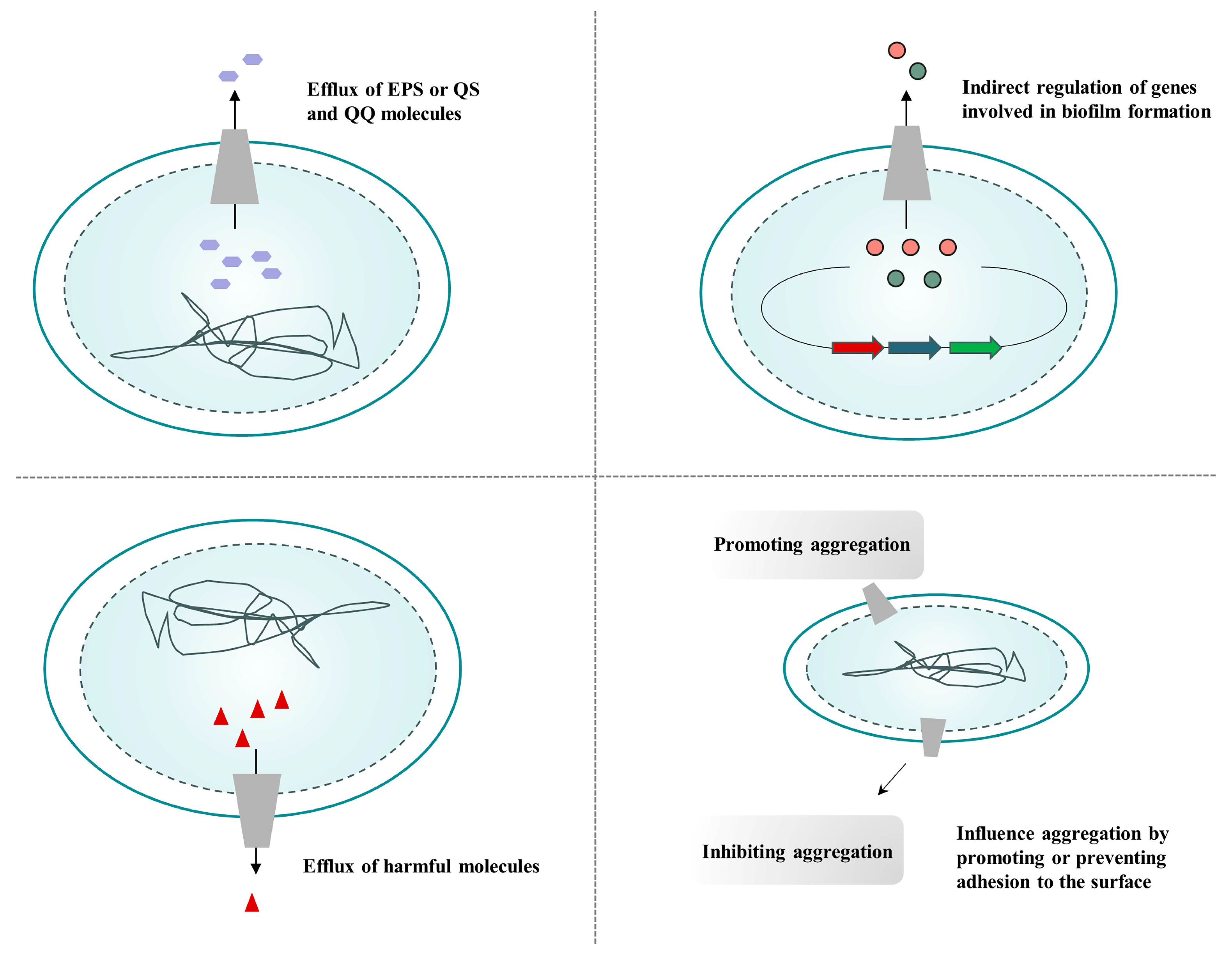
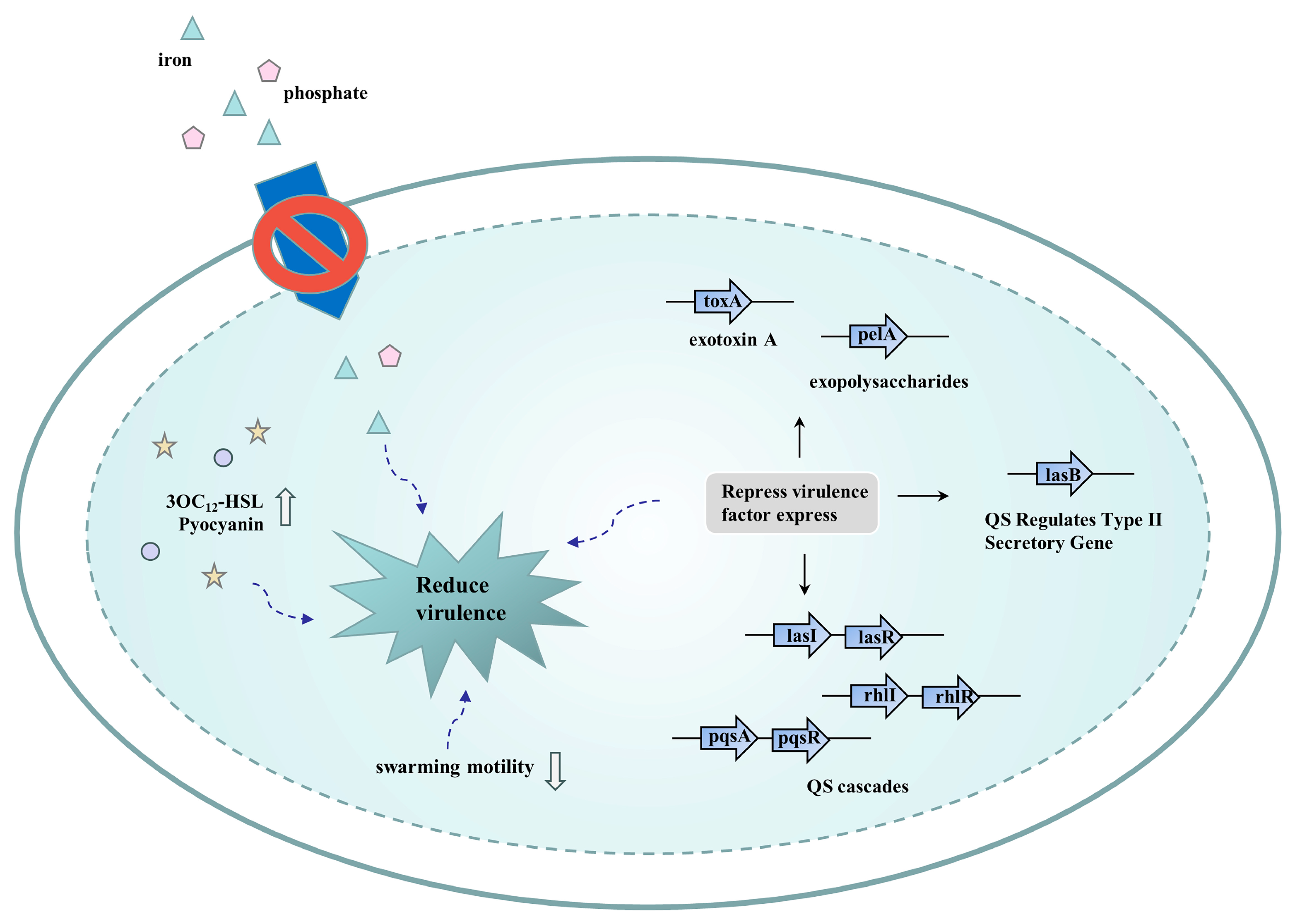
4.2. Efflux Pump Inhibitors Reduce Bacterial Virulence
4.3. Efflux Pump Inhibitors Reduce the Formation of Bacterial Persister Cells
Consequently, the combination of efflux pump inhibitors with conventional antibiotics holds promise for the treatment of persistent bacterial disease by reducing intrinsic resistance or acquired multidrug resistance associated with efflux pumps. These compounds assist antibiotics to clear persister cells, thereby ultimately killing the pathogenic microbial population.
4.4. Efflux Pump Inhibitors Limit the Acquisition of Bacterial Resistance
4.5.Efflux Pump Inhibitors Improve Mismatch Repair
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics16020170
References
- Lei, H.; Wang, C.; Wang, Y.; Wang, C. Single-cell RNA-Seq revealed profound immune alteration in the peripheral blood of patients with bacterial infection. Int. J. Infect. Dis. 2021, 103, 527–535.
- Kovalchuk, O.; Walz, P.; Kovalchuk, I. Does bacterial infection cause genome instability and cancer in the host cell? Mutat. Res. 2014, 761, 1–14.
- García-Castro, M.; Sarabia, F.; Díaz-Morilla, A.; López-Romero, J.M. Approved antibacterial drugs in the last 10 years: From the bench to the clinic. Explor. Drug Sci. 2023, 1, 180–209.
- Allcock, S.; Young, E.H.; Holmes, M.; Gurdasani, D.; Dougan, G.; Sandhu, M.S.; Solomon, L.; Török, M.E. Antimicrobial resistance in human populations: Challenges and opportunities. Glob. Health Epidemiol. Genom. 2017, 2, e4.
- Hancock, R.E. The role of fundamental research and biotechnology in finding solutions to the global problem of antibiotic resistance. Clin. Infect. Dis. 1997, 24 (Suppl. S1), S148–S150.
- Lamut, A.; Peterlin Mašič, L.; Kikelj, D.; Tomašič, T. Efflux pump inhibitors of clinically relevant multidrug resistant bacteria. Med. Res. Rev. 2019, 39, 2460–2504.
- Laws, M.; Jin, P.; Rahman, K.M. Efflux pumps in Mycobacterium tuberculosis and their inhibition to tackle antimicrobial resistance. Trends Microbiol. 2022, 30, 57–68.
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, 10–1128.
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107.
- Monteiro, K.L.C.; de Aquino, T.M.; Mendonça Junior, F.J.B. An Update on Staphylococcus aureus NorA Efflux Pump Inhibitors. Curr. Top. Med. Chem. 2020, 20, 2168–2185.
- Liao, W.; Liu, Y.; Zhang, W. Virulence evolution, molecular mechanisms of resistance and prevalence of ST11 carbapenem-resistant Klebsiella pneumoniae in China: A review over the last 10 years. J. Glob. Antimicrob. Resist. 2020, 23, 174–180.
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192.
- von Baum, H.; Marre, R. Antimicrobial resistance of Escherichia coli and therapeutic implications. Int. J. Med. Microbiol. 2005, 295, 503–511.
- Iovine, N.M. Resistance mechanisms in Campylobacter jejuni. Virulence 2013, 4, 230–240.
- Mahmood, H.Y.; Jamshidi, S.; Sutton, J.M.; Rahman, K.M. Current Advances in Developing Inhibitors of Bacterial Multidrug Efflux Pumps. Curr. Med. Chem. 2016, 23, 1062–1081.
- AlMatar, M.; Albarri, O.; Makky, E.A.; Köksal, F. Efflux pump inhibitors: New updates. Pharmacol. Rep. 2021, 73, 1–16.
- Jamshidi, S.; Sutton, J.M.; Rahman, K.M. An overview of bacterial efflux pumps and computational approaches to study efflux pump inhibitors. Future Med. Chem. 2016, 8, 195–210.
- Schindler, B.D.; Kaatz, G.W. Multidrug efflux pumps of Gram-positive bacteria. Drug Resist. Updat. 2016, 27, 1–13.
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539.
- Marquez, B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie 2005, 87, 1137–1147.
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019, 149, 129–145.
- Mahey, N.; Tambat, R.; Chandal, N.; Verma, D.K.; Thakur, K.G.; Nandanwar, H. Repurposing Approved Drugs as Fluoroquinolone Potentiators to Overcome Efflux Pump Resistance in Staphylococcus aureus. Microbiol. Spectr. 2021, 9, e0095121.
- Pereira da Cruz, R.; Sampaio de Freitas, T.; Socorro Costa, M.d.; Lucas Dos Santos, A.T.; Ferreira Campina, F.; Pereira, R.L.S.; Bezerra, J.W.A.; Quintans-Júnior, L.J.; De Souza Araújo, A.A.; Júnior, J.P.D.S.; et al. Effect of α-Bisabolol and Its β-Cyclodextrin Complex as TetK and NorA Efflux Pump Inhibitors in Staphylococcus aureus Strains. Antibiotics 2020, 9, 28.
- Zimmermann, S.; Klinger-Strobel, M.; Bohnert, J.A.; Wendler, S.; Rödel, J.; Pletz, M.W.; Löffler, B.; Tuchscherr, L. Clinically Approved Drugs Inhibit the Staphylococcus aureus Multidrug NorA Efflux Pump and Reduce Biofilm Formation. Front. Microbiol. 2019, 10, 2762.
- Xu, Q.; Sheng, Z.; Hao, M.; Jiang, J.; Ye, M.; Chen, Y.; Xu, X.; Guo, Q.; Wang, M. RamA upregulates multidrug resistance efflux pumps AcrAB and OqxAB in Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2021, 57, 106251.
- Blair, J.M.A.; Richmond, G.E.; Piddock, L.J.V. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol. 2014, 9, 1165–1177.
- Schuster, S.; Vavra, M.; Schweigger, T.M.; Rossen, J.W.A.; Matsumura, Y.; Kern, W.V. Contribution of AcrAB-TolC to multidrug resistance in an Escherichia coli sequence type 131 isolate. Int. J. Antimicrob. Agents 2017, 50, 477–481.
- Guo, B.; Lin, J.; Reynolds, D.L.; Zhang, Q. Contribution of the multidrug efflux transporter CmeABC to antibiotic resistance in different Campylobacter species. Foodborne Pathog. Dis. 2010, 7, 77–83.
- Jeon, B.; Zhang, Q. Sensitization of Campylobacter jejuni to fluoroquinolone and macrolide antibiotics by antisense inhibition of the CmeABC multidrug efflux transporter. J. Antimicrob. Chemother. 2009, 63, 946–948.
- Sharifi, S.; Bakhshi, B.; Najar-Peerayeh, S. Significant contribution of the CmeABC Efflux pump in high-level resistance to ciprofloxacin and tetracycline in Campylobacter jejuni and Campylobacter coli clinical isolates. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 36.
- Blanco, P.; Sanz-García, F.; Hernando-Amado, S.; Martínez, J.L.; Alcalde-Rico, M. The development of efflux pump inhibitors to treat Gram-negative infections. Expert Opin. Drug Discov. 2018, 13, 919–931.
- Stavri, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260.
- Giorgini, G.; Mangiaterra, G.; Cedraro, N.; Laudadio, E.; Sabbatini, G.; Cantarini, M.; Minnelli, C.; Mobbili, G.; Frangipani, E.; Biavasco, F.; et al. Berberine Derivatives as Pseudomonas aeruginosa MexXY-OprM Inhibitors: Activity and In Silico Insights. Molecules 2021, 26, 6644.
- Khan, I.A.; Mirza, Z.M.; Kumar, A.; Verma, V.; Qazi, G.N. Piperine, a phytochemical potentiator of ciprofloxacin against Staphylococcus aureus. Antimicrob. Agents Chemother. 2006, 50, 810–812.
- Mirza, Z.M.; Kumar, A.; Kalia, N.P.; Zargar, A.; Khan, I.A. Piperine as an inhibitor of the MdeA efflux pump of Staphylococcus aureus. J. Med. Microbiol. 2011, 60, 1472–1478.
- Sharma, S.; Kumar, M.; Sharma, S.; Nargotra, A.; Koul, S.; Khan, I.A. Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2010, 65, 1694–1701.
- Chan, B.C.L.; Ip, M.; Lau, C.B.S.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773.
- Holler, J.G.; Christensen, S.B.; Slotved, H.-C.; Rasmussen, H.B.; Gúzman, A.; Olsen, C.-E.; Petersen, B.; Mølgaard, P. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from Persea lingue Nees. J. Antimicrob. Chemother. 2012, 67, 1138–1144.
- Freitas, T.S.; Xavier, J.C.; Pereira, R.L.S.; Rocha, J.E.; Campina, F.F.; de Araújo Neto, J.B.; Silva, M.M.C.; Barbosa, C.R.S.; Marinho, E.S.; Nogueira, C.E.S.; et al. In vitro and in silico studies of chalcones derived from natural acetophenone inhibitors of NorA and MepA multidrug efflux pumps in Staphylococcus aureus. Microb. Pathog. 2021, 161, 105286.
- Guo, Y.; Huang, C.; Su, H.; Zhang, Z.; Chen, M.; Wang, R.; Zhang, D.; Zhang, L.; Liu, M. Luteolin increases susceptibility to macrolides by inhibiting MsrA efflux pump in Trueperella pyogenes. Vet. Res. 2022, 53, 3.
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5’-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437.
- Kanagaratnam, R.; Sheikh, R.; Alharbi, F.; Kwon, D.H. An efflux pump (MexAB-OprM) of Pseudomonas aeruginosa is associated with antibacterial activity of Epigallocatechin-3-gallate (EGCG). Phytomedicine 2017, 36, 194–200.
- Sudano Roccaro, A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973.
- Opperman, T.J.; Kwasny, S.M.; Kim, H.-S.; Nguyen, S.T.; Houseweart, C.; D’Souza, S.; Walker, G.C.; Peet, N.P.; Nikaido, H.; Bowlin, T.L. Characterization of a novel pyranopyridine inhibitor of the AcrAB efflux pump of Escherichia coli. Antimicrob. Agents Chemother. 2014, 58, 722–733.
- Tohidpour, A.; Najar Peerayeh, S.; Mehrabadi, J.F.; Rezaei Yazdi, H. Determination of the efflux pump-mediated resistance prevalence in Pseudomonas aeruginosa, using an efflux pump inhibitor. Curr. Microbiol. 2009, 59, 352–355.
- Bailey, A.M.; Paulsen, I.T.; Piddock, L.J.V. RamA confers multidrug resistance in Salmonella enterica via increased expression of acrB, which is inhibited by chlorpromazine. Antimicrob. Agents Chemother. 2008, 52, 3604–3611.
- Pannek, S.; Higgins, P.G.; Steinke, P.; Jonas, D.; Akova, M.; Bohnert, J.A.; Seifert, H.; Kern, W.V. Multidrug efflux inhibition in Acinetobacter baumannii: Comparison between 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-beta-naphthylamide. J. Antimicrob. Chemother. 2006, 57, 970–974.
- Nakashima, R.; Sakurai, K.; Yamasaki, S.; Hayashi, K.; Nagata, C.; Hoshino, K.; Onodera, Y.; Nishino, K.; Yamaguchi, A. Structural basis for the inhibition of bacterial multidrug exporters. Nature 2013, 500, 102–106.
- Kalia, N.P.; Mahajan, P.; Mehra, R.; Nargotra, A.; Sharma, J.P.; Koul, S.; Khan, I.A. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J. Antimicrob. Chemother. 2012, 67, 2401–2408.
- Zhang, Y.; Huang, Z.; Omari-Siaw, E.; Lu, S.; Zhu, Y.; Jiang, D.; Wang, M.; Yu, J.; Xu, X.; Zhang, W. Preparation and In Vitro-In Vivo Evaluation of Sustained-Release Matrix Pellets of Capsaicin to Enhance the Oral Bioavailability. AAPS PharmSciTech 2016, 17, 339–349.
- Naaz, F.; Khan, A.; Kumari, A.; Ali, I.; Ahmad, F.; Ahmad Lone, B.; Ahmad, N.; Ali Khan, I.; Rajput, V.S.; Grover, A.; et al. 1,3,4-oxadiazole conjugates of capsaicin as potent NorA efflux pump inhibitors of Staphylococcus aureus. Bioorg. Chem. 2021, 113, 105031.
- Azam, S.; Park, J.-Y.; Kim, I.-S.; Choi, D.-K. Piperine and Its Metabolite’s Pharmacology in Neurodegenerative and Neurological Diseases. Biomedicines 2022, 10, 154.
- Deka, B.; Suri, M.; Sarma, S.; Devi, M.V.; Bora, A.; Sen, T.; Dihingia, A.; Pahari, P.; Singh, A.K. Potentiating the intracellular killing of Staphylococcus aureus by dihydroquinazoline analogues as NorA efflux pump inhibitor. Bioorg. Med. Chem. 2022, 54, 116580.
- Bharatham, N.; Bhowmik, P.; Aoki, M.; Okada, U.; Sharma, S.; Yamashita, E.; Shanbhag, A.P.; Rajagopal, S.; Thomas, T.; Sarma, M.; et al. Structure and function relationship of OqxB efflux pump from Klebsiella pneumoniae. Nat. Commun. 2021, 12, 5400.
- Hasdemir, U.O.; Chevalier, J.; Nordmann, P.; Pagès, J.-M. Detection and prevalence of active drug efflux mechanism in various multidrug-resistant Klebsiella pneumoniae strains from Turkey. J. Clin. Microbiol. 2004, 42, 2701–2706.
- Sun, L.; Sun, L.; Li, X.; Hu, X.; Wang, X.; Nie, T.; Zhang, Y.; You, X. A Novel Tigecycline Adjuvant ML-7 Reverses the Susceptibility of Tigecycline-Resistant Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2021, 11, 809542.
- Chevalier, J.; Bredin, J.; Mahamoud, A.; Malléa, M.; Barbe, J.; Pagès, J.-M. Inhibitors of antibiotic efflux in resistant Enterobacter aerogenes and Klebsiella pneumoniae strains. Antimicrob. Agents Chemother. 2004, 48, 1043–1046.
- Mahamoud, A.; Chevalier, J.; Baitiche, M.; Adam, E.; Pagès, J.-M. An alkylaminoquinazoline restores antibiotic activity in Gram-negative resistant isolates. Microbiology 2011, 157, 566–571.
- Chevalier, J.; Mahamoud, A.; Baitiche, M.; Adam, E.; Viveiros, M.; Smarandache, A.; Militaru, A.; Pascu, M.L.; Amaral, L.; Pagès, J.-M. Quinazoline derivatives are efficient chemosensitizers of antibiotic activity in Enterobacter aerogenes, Klebsiella pneumoniae and Pseudomonas aeruginosa resistant strains. Int. J. Antimicrob. Agents 2010, 36, 164–168.
- Yan, S.; Chang, J.; Hao, X.; Liu, J.; Tan, X.; Geng, Z.; Wang, Z. Berberine regulates short-chain fatty acid metabolism and alleviates the colitis-associated colorectal tumorigenesis through remodeling intestinal flora. Phytomedicine 2022, 102, 154217.
- Feng, X.; Wang, K.; Cao, S.; Ding, L.; Qiu, F. Pharmacokinetics and Excretion of Berberine and Its Nine Metabolites in Rats. Front. Pharmacol. 2020, 11, 594852.
- Zhou, X.-Y.; Ye, X.-G.; He, L.-T.; Zhang, S.-R.; Wang, R.-L.; Zhou, J.; He, Z.-S. In vitro characterization and inhibition of the interaction between ciprofloxacin and berberine against multidrug-resistant Klebsiella pneumoniae. J. Antibiot. 2016, 69, 741–746.
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167.
- Pal, A.; Tripathi, A. Quercetin inhibits carbapenemase and efflux pump activities among carbapenem-resistant Gram-negative bacteria. APMIS 2020, 128, 251–259.
- Cheng, Y.; Liu, Y.; Chen, D.; Zhou, Y.; Yu, S.; Lin, H.; Liao, C.K.; Lin, H.; Xu, P.; Huang, M. Dual effects of quercetin on protein digestion and absorption in the digestive tract. Food Chem. 2021, 358, 129891.
- Pokharel, K.; Dawadi, B.R.; Bhatt, C.P.; Gupte, S. Prevalence of Pseudomonas Aeruginosa and its Antibiotic Sensitivity Pattern. J. Nepal Health Res. Counc. 2019, 17, 109–113.
- Sala, A.; Di Ianni, F.; Pelizzone, I.; Bertocchi, M.; Santospirito, D.; Rogato, F.; Flisi, S.; Spadini, C.; Iemmi, T.; Moggia, E.; et al. The prevalence of Pseudomonas aeruginosa and multidrug resistant Pseudomonas aeruginosa in healthy captive ophidian. PeerJ 2019, 7, e6706.
- Gan, R.-Y.; Li, H.-B.; Sui, Z.-Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941.
- Zhang, S.; Mao, B.; Cui, S.; Zhang, Q.; Zhao, J.; Tang, X.; Chen, W. Absorption, metabolism, bioactivity, and biotransformation of epigallocatechin gallate. Crit. Rev. Food Sci. Nutr. 2023, 1–21.
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent Advances in Biotransformation of Saponins. Molecules 2019, 24, 2365.
- Hazlett, L.D.; Ekanayaka, S.A.; McClellan, S.A.; Francis, R. Glycyrrhizin Use for Multi-Drug Resistant Pseudomonas aeruginosa: In Vitro and In Vivo Studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2978–2989.
- Tambat, R.; Mahey, N.; Chandal, N.; Verma, D.K.; Jangra, M.; Thakur, K.G.; Nandanwar, H. A Microbe-Derived Efflux Pump Inhibitor of the Resistance-Nodulation-Cell Division Protein Restores Antibiotic Susceptibility in Escherichia coli and Pseudomonas aeruginosa. ACS Infect. Dis. 2022, 8, 255–270.
- Yuan, Y.; Rosado-Lugo, J.D.; Zhang, Y.; Datta, P.; Sun, Y.; Cao, Y.; Banerjee, A.; Parhi, A.K. Evaluation of Heterocyclic Carboxamides as Potential Efflux Pump Inhibitors in Pseudomonas aeruginosa. Antibiotics 2021, 11, 30.
- Zhang, Y.; Rosado-Lugo, J.D.; Datta, P.; Sun, Y.; Cao, Y.; Banerjee, A.; Yuan, Y.; Parhi, A.K. Evaluation of a Conformationally Constrained Indole Carboxamide as a Potential Efflux Pump Inhibitor in Pseudomonas aeruginosa. Antibiotics 2022, 11, 716.
- Abd El-Tawab, A.A.; Ammar, A.M.; Ahmed, H.A.; Hefny, A.A. Efflux Pump Inhibitors, Alpha-Tocopherol and Aspirin: Role in Campylobacter jejuni and Campylobacter coli Fluoroquinolone Resistance. Microb. Drug Resist. 2019, 25, 203–211.
- Zeng, B.; Wang, H.; Zou, L.; Zhang, A.; Yang, X.; Guan, Z. Evaluation and target validation of indole derivatives as inhibitors of the AcrAB-TolC efflux pump. Biosci. Biotechnol. Biochem. 2010, 74, 2237–2241.
- Cui, Z.-H.; He, H.-L.; Zheng, Z.-J.; Yuan, Z.-Q.; Chen, Y.; Huang, X.-Y.; Ren, H.; Zhou, Y.-F.; Zhao, D.-H.; Fang, L.-X.; et al. Phentolamine Significantly Enhances Macrolide Antibiotic Antibacterial Activity against MDR Gram-Negative Bacteria. Antibiotics 2023, 12, 760.
- Lu, W.-J.; Hsu, P.-H.; Chang, C.-J.; Su, C.-K.; Huang, Y.-J.; Lin, H.-J.; Lai, M.; Ooi, G.-X.; Dai, J.-Y.; Lin, H.-T.V. Identified Seaweed Compound Diphenylmethane Serves as an Efflux Pump Inhibitor in Drug-Resistant Escherichia coli. Antibiotics 2021, 10, 1378.
- García-Sánchez, L.; Melero, B.; Jaime, I.; Rossi, M.; Ortega, I.; Rovira, J. Biofilm formation, virulence and antimicrobial resistance of different Campylobacter jejuni isolates from a poultry slaughterhouse. Food Microbiol. 2019, 83, 193–199.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282.
- Oh, E.; Jeon, B. Synergistic anti-Campylobacter jejuni activity of fluoroquinolone and macrolide antibiotics with phenolic compounds. Front. Microbiol. 2015, 6, 1129.
- Kovač, J.; Šimunović, K.; Wu, Z.; Klančnik, A.; Bucar, F.; Zhang, Q.; Možina, S.S. Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLoS ONE 2015, 10, e0122871.
- Martinez, A.L.; Lin, J. Effect of an efflux pump inhibitor on the function of the multidrug efflux pump CmeABC and antimicrobial resistance in Campylobacter. Foodborne Pathog. Dis. 2006, 3, 393–402.
- Kumar, A.; Ito, A.; Hirohama, M.; Yoshida, M.; Zhang, K.Y.J. Identification of new SUMO activating enzyme 1 inhibitors using virtual screening and scaffold hopping. Bioorg. Med. Chem. Lett. 2016, 26, 1218–1223.
- Ke, Y.-Y.; Coumar, M.S.; Shiao, H.-Y.; Wang, W.-C.; Chen, C.-W.; Song, J.-S.; Chen, C.-H.; Lin, W.-H.; Wu, S.-H.; Hsu, J.T.A.; et al. Ligand efficiency based approach for efficient virtual screening of compound libraries. Eur. J. Med. Chem. 2014, 83, 226–235.
- Brown, A.R.; Ettefagh, K.A.; Todd, D.; Cole, P.S.; Egan, J.M.; Foil, D.H.; Graf, T.N.; Schindler, B.D.; Kaatz, G.W.; Cech, N.B. A mass spectrometry-based assay for improved quantitative measurements of efflux pump inhibition. PLoS ONE 2015, 10, e0124814.
- Elhidar, N.; Nafis, A.; Goehler, A.; Abbad, A.; Hassani, L.; Mezrioui, N.-E.; Bohnert, J.A. Novel DiOC3 96-well real-time efflux assay for discovery of NorA efflux pump inhibitors in Staphylococcus aureus. J. Microbiol. Methods 2021, 181, 106128.
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332.
- Reza, A.; Sutton, J.M.; Rahman, K.M. Effectiveness of Efflux Pump Inhibitors as Biofilm Disruptors and Resistance Breakers in Gram-Negative (ESKAPEE) Bacteria. Antibiotics 2019, 8, 229.
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020.
- Kvist, M.; Hancock, V.; Klemm, P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl. Environ. Microbiol. 2008, 74, 7376–7382.
- Singh, S.; Kalia, N.P.; Joshi, P.; Kumar, A.; Sharma, P.R.; Kumar, A.; Bharate, S.B.; Khan, I.A. Boeravinone B, A Novel Dual Inhibitor of NorA Bacterial Efflux Pump of Staphylococcus aureus and Human P-Glycoprotein, Reduces the Biofilm Formation and Intracellular Invasion of Bacteria. Front. Microbiol. 2017, 8, 1868.
- Xu, C.; Dong, N.; Chen, K.; Yang, X.; Zeng, P.; Hou, C.; Chi Chan, E.W.; Yao, X.; Chen, S. Bactericidal, anti-biofilm, and anti-virulence activity of vitamin C against carbapenem-resistant hypervirulent Klebsiella pneumoniae. iScience 2022, 25, 103894.
- Brannon, J.R.; Hadjifrangiskou, M. The arsenal of pathogens and antivirulence therapeutic strategies for disarming them. Drug Des. Devel Ther. 2016, 10, 1795–1806.
- Rampioni, G.; Pillai, C.R.; Longo, F.; Bondì, R.; Baldelli, V.; Messina, M.; Imperi, F.; Visca, P.; Leoni, L. Effect of efflux pump inhibition on Pseudomonas aeruginosa transcriptome and virulence. Sci. Rep. 2017, 7, 11392.
- El-Shaer, S.; Shaaban, M.; Barwa, R.; Hassan, R. Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl β-naphthylamide. J. Med. Microbiol. 2016, 65, 1194–1204.
- Bina, X.R.; Philippart, J.A.; Bina, J.E. Effect of the efflux inhibitors 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-beta-naphthylamide on antimicrobial susceptibility and virulence factor production in Vibrio cholerae. J. Antimicrob. Chemother. 2009, 63, 103–108.
- Pu, Y.; Zhao, Z.; Li, Y.; Zou, J.; Ma, Q.; Zhao, Y.; Ke, Y.; Zhu, Y.; Chen, H.; Baker, M.A.B.; et al. Enhanced Efflux Activity Facilitates Drug Tolerance in Dormant Bacterial Cells. Mol. Cell. 2016, 62, 284–294.
- Chang, J.; Lee, R.-E.; Lee, W. A pursuit of Staphylococcus aureus continues: A role of persister cells. Arch. Pharm. Res. 2020, 43, 630–638.
- Byrd, B.A.; Zenick, B.; Rocha-Granados, M.C.; Englander, H.E.; Hare, P.J.; LaGree, T.J.; DeMarco, A.M.; Mok, W.W.K. The AcrAB-TolC Efflux Pump Impacts Persistence and Resistance Development in Stationary-Phase Escherichia coli following Delafloxacin Treatment. Antimicrob. Agents Chemother. 2021, 65, e0028121.
- Povolo, V.R.; Ackermann, M. Disseminating antibiotic resistance during treatment. Science 2019, 364, 737–738.
- Nolivos, S.; Cayron, J.; Dedieu, A.; Page, A.; Delolme, F.; Lesterlin, C. Role of AcrAB-TolC multidrug efflux pump in drug-resistance acquisition by plasmid transfer. Science 2019, 364, 778–782.
- Busseti, M.I.I.; Margara, L.M.; Castell, S.D.; Fernández, M.M.; Malchiodi, E.L.; Montich, G.G.; Miguel, V.; Argaraña, C.E.; Monti, M.R. MutS recognition of mismatches within primed DNA replication intermediates. DNA Repair 2022, 119, 103392.
- El Meouche, I.; Dunlop, M.J. Heterogeneity in efflux pump expression predisposes antibiotic-resistant cells to mutation. Science 2018, 362, 686–690.
