In situations where animal models (AMs) are necessary, as in the field of neuroscience, a strong culture of care must be supported and established. The pivotal question remains: how can we uphold a robust “culture of care”? In the multifaceted domain of neuroscience research, AMs traverse a spectrum shaped by conflicting viewpoints, anthropocentrism and pathocentrism, where established scientific norms intersect with ethical deliberations. Anthropocentrism, representative of conventional scientific approaches, may prioritize scientific goals potentially to the detriment of animal welfare. Conversely, pathocentrism places significant importance on the ethical treatment and well-being of AMs. This divergence of approach prompts the imperative development of a robust culture of care framework within research institutions, advocating for animal welfare, ethical responsibility, and adherence to regulatory standards. In this review, we refer to a European view of animal care, discussing internationally valid concepts that find rebuttal in the current European legislation. This review meticulously analyzes the many facets of the culture of care, particularly for neuroscience studies involving AMs, illustrating the principles, practices, and collaborations critical to overcoming ethical expectations. This commitment increases credibility and builds trust in the public and research spheres, underscoring the critical importance of a culture of care in the ethics of neuroscience research.
- animal welfare
- 3Rs
- ethical responsibility
- research community
- neuroscience research
Ethical Considerations: Prioritizing Animal Welfare and Scientific Progress
Ethical Paradigms: Anthropocentrism and Pathocentrism Examined
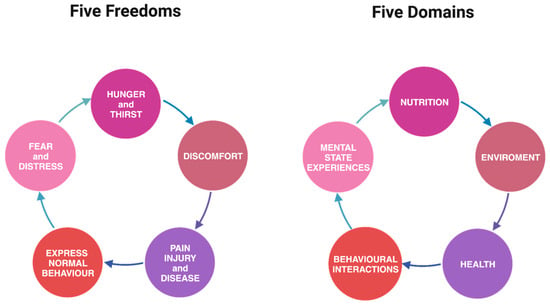
Synergizing Ethical Compasses: Comparing the Five Freedoms and the Five Domains
This entry is adapted from the peer-reviewed paper 10.3390/encyclopedia4010018
References
- Lambert, K. Wild brains: The value of neuroethological approaches in preclinical behavioral neuroscience animal models. Neurosci. Biobehav. Rev. 2023, 146, 105044.
- Romanova, E.V.; Sweedler, J.V. Animal model systems in neuroscience. ACS Chem. Neurosci. 2018, 9, 1869–1870.
- Bovenkerk, B.; Kaldewaij, F. The use of animal models in behavioural neuroscience research. Curr. Top. Behav. Neurosci. 2015, 19, 17–46.
- Crystal, J.D. Elements of episodic-like memory in animal models. Behav. Process. 2009, 80, 269–277.
- Yanshree; Yu, W.S.; Fung, M.L.; Lee, C.W.; Lim, L.W.; Wong, K.H. The monkey head mushroom and memory enhancement in Alzheimer’s disease. Cells 2022, 11, 2284.
- Fine, A.H.; Beck, A.M.; Ng, Z. The State of Animal-Assisted Interventions: Addressing the Contemporary Issues that will Shape the Future. Int. J. Environ. Res. Public Health 2019, 16, 3997.
- DeGrazia, D.; Beauchamp, T.L. Beyond the 3Rs to a more comprehensive framework of principles for animal research ethics. ILAR J. 2021, 60, 308–317.
- Gruen, L. Ethics and Animals: An Introduction; Cambridge University Press: Cambridge, UK, 2021; ISBN 9781108988544.
- Robinson, S.; Sparrow, S.; Williams, B.; Decelle, T.; Bertelsen, T.; Reid, K.; Chlebus, M. The European Federation of the Pharmaceutical Industry and Associations’ Research and Animal Welfare Group: Assessing and benchmarking “Culture of Care” in the context of using animals for scientific purpose. Lab. Anim. 2019, 54, 23677219887998.
- Arndt, S.S.; Goerlich, V.C.; van der Staay, F.J. A dynamic concept of animal welfare: The role of appetitive and adverse internal and external factors and the animal’s ability to adapt to them. Front. Anim. Sci. 2022, 3.
- Davies, G.; Gorman, R.; Greenhough, B.; Hobson-West, P.; Kirk, R.G.W.; Message, R.; Myelnikov, D.; Palmer, A.; Roe, E.; Ashall, V.; et al. Animal research nexus: A new approach to the connections between science, health and animal welfare. Med. Humanit. 2020, 46, 499–511.
- Regan, T. Animal Rights, Human Wrongs: An Introduction to Moral Philosophy; Rowman & Littlefield Publishers: Lanham, MD, USA, 2003; ISBN 9780742599383.
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011; ISBN 0309154006.
- Ferrara, F.; Hiebl, B.; Kunzmann, P.; Hutter, F.; Afkham, F.; LaFollette, M.; Gruber, C. Culture of care in animal research—Expanding the 3Rs to include people. Lab. Anim. 2022, 56, 511–518.
- Soulsbury, C.; Gray, H.; Smith, L.; Braithwaite, V.; Cotter, S.; Elwood, R.W.; Wilkinson, A.; Collins, L.M. The welfare and ethics of research involving wild animals: A primer. Methods Ecol. Evol. 2020, 11, 1164–1181.
- Wahyuwardani, S.; Noor, S.M.; Bakrie, B. Animal welfare ethics in research and testing: Implementation and its barrier. WARTAZOA 2020, 30, 211.
- Lee, K.H.; Lee, D.W.; Kang, B.C. The “R” principles in laboratory animal experiments. Lab. Anim. Res. 2020, 36, 45.
- Blumer, K. Ethical aspects of animal experiments and the principle of solidarity. In Deutsche Forschungsgemeinschaft (DFG). Animal Experiments in Research; Exner, C., Bode, H.-J., Blumer, C., Giese, C., Eds.; Lemmens Medien: Bonn, Germany, 2007.
- Martinez, J.; von Nolting, C. Review: “Animal welfare”—A European concept. Animal 2023, 17 (Suppl. 4), 100839.
- Maple, T.L.; Bloomsmith, M.A. Introduction: The science and practice of optimal animal welfare. Behav. Process. 2018, 156, 1–2.
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141.
- National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research. Guidance for the Description of Animal Research in Scientific Publications; National Academies Press: Washington, DC, USA, 2011; ISBN 9780309219518.
- Garner, J.P. The significance of meaning: Why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? ILAR J. 2014, 55, 438–456.
- Coscas, R.; Senemaud, J. Experimenters or Amateurs? Eur. J. Vasc. Endovasc. Surg. 2020, 60, 253.
- van der Velden, J.; Asselbergs, F.W.; Bakkers, J.; Batkai, S.; Bertrand, L.; Bezzina, C.R.; Bot, I.; Brundel, B.J.J.M.; Carrier, L.; Chamuleau, S.; et al. Animal models and animal-free innovations for cardiovascular research: Current status and routes to be explored. Consensus document of the ESC Working Group on Myocardial Function and the ESC Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2022, 118, 3016–3051.
- Kurtz, D.M.; Feeney, W.P. The influence of feed and drinking water on terrestrial animal research and study replicability. ILAR J. 2020, 60, 175–196.
- Brown, M.J.; Symonowicz, C.; Medina, L.V.; Bratcher, N.A.; Buckmaster, C.A.; Klein, H.; Anderson, L.C. Culture of care: Organizational responsibilities. In Management of Animal Care and Use Programs in Research, Education, and Testing; Weichbrod, R.H., Thompson, G.A., Norton, J.N., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; ISBN 9781315152189.
- Bertelsen, T.; Øvlisen, K. Assessment of the Culture of Care working with laboratory animals by using a comprehensive survey tool. Lab. Anim. 2021, 55, 453–462.
- Williams, A. Caring for those who care: Towards a more expansive understanding of ‘cultures of care’ in laboratory animal facilities. Soc. Cult. Geogr. 2023, 24, 31–48.
- Hubrecht, R.C.; Carter, E. The 3Rs and humane experimental technique: Implementing change. Animals 2019, 9, 754.
- Buchheister, S.; Bleich, A. Health Monitoring of Laboratory Rodent Colonies-Talking about (R)evolution. Animals 2021, 11, 1410.
- Wu, J. Landscape sustainability science: Ecosystem services and human well-being in changing landscapes. Landsc. Ecol. 2013, 28, 999–1023.
- Liz Paola, N.Z.; Torgerson, P.R.; Hartnack, S. Alternative paradigms in animal health decisions: A framework for treating animals not only as commodities. Animals 2022, 12, 1845.
- Kopnina, H.; Washington, H.; Taylor, B.; Piccolo, J.J. Anthropocentrism: More than Just a Misunderstood Problem. J. Agric. Environ. Ethics 2018, 31, 109–127.
- Croney, C.C.; Anthony, R. Engaging science in a climate of values: Tools for animal scientists tasked with addressing ethical problems. J. Anim. Sci. 2010, 88, E75–E81.
- Beausoleil, N.J. I am a compassionate conservation welfare scientist: Considering the theoretical and practical differences between compassionate conservation and conservation welfare. Animals 2020, 10, 257.
- Eggel, M.; Camenzind, S. Authorization of animal research proposals—A comparison of harm concepts in different European regulations. Berl. Münchener Tierärztliche Wochenschr. 2020. online first.
- Baertschi, B.; Gyger, M. Ethical considerations in mouse experiments. Curr. Protoc. Mouse Biol. 2011, 1, 155–167.
- Gross, D.; Tolba, R.H. Ethics in Animal-Based Research. Eur. Surg. Res. 2015, 55, 43–57.
- Grimm, H. Ethics in laboratory animal science. In Comparative Medicine; Jensen-Jarolim, E., Ed.; Springer: Vienna, Austria, 2014; pp. 281–300. ISBN 978-3-7091-1558-9.
- Millar, K.M. Translational stem cell research and animal use: Examining ethical issues and opportunities. In Translational Stem Cell Research; Hug, K., Hermerén, G., Eds.; Stem Cell Biology and Regenerative Medicine; Humana Press: Totowa, NJ, USA, 2011; pp. 113–124. ISBN 978-1-60761-958-1.
- Vorstenbosch, J.M.G. The ethics of the Three Rs principle: A reconsideration. Anim. Welf. 2005, 14, 339–345.
- Grunwald, A. Living Technology: Philosophy and Ethics at the Crossroads between Life and Technology; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781000346428.
- Schindler, S. The animal’s dignity in Swiss Animal Welfare Legislation—Challenges and opportunities. Eur. J. Pharm. Biopharm. 2013, 84, 251–254.
- Schmidt, K. Concepts of animal welfare in relation to positions in animal ethics. Acta Biotheor. 2011, 59, 153–171.
- Mannhold, R.; Kubinyi, H.; Folkers, G. Animal Models for Human Cancer: Discovery and Development of Novel Therapeutics; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9783527339976.
- Arlinghaus, R.; Cooke, S.J.; Lyman, J.; Policansky, D.; Schwab, A.; Suski, C.; Sutton, S.G.; Thorstad, E.B. Understanding the Complexity of Catch-and-Release in Recreational Fishing: An Integrative Synthesis of Global Knowledge from Historical, Ethical, Social, and Biological Perspectives. Rev. Fish. Sci. 2007, 15, 75–167.
- McCausland, C. The five freedoms of animal welfare are rights. J. Agric. Environ. Ethics 2014, 27, 649–662.
- Mellor, D.J. Moving beyond the “Five Freedoms” by Updating the “Five Provisions” and Introducing Aligned “Animal Welfare Aims”. Animals 2016, 6, 59.
- Serpell, J.A.; Coppinger, R.; Fine, A.H.; Peralta, J.M. Welfare considerations in therapy and assistance animals. In Handbook on Animal-Assisted Therapy; Elsevier: Amsterdam, The Netherlands, 2010; pp. 481–503. ISBN 9780123814531.
- Jaasma, L. A Review of the Housing Conditions for Laboratory Animals. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, July 2014.
- Gregory, N.G. Physiology and Behaviour of Animal Suffering; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9781405173025.
- Mellor, D.J. Operational details of the five domains model and its key applications to the assessment and management of animal welfare. Animals 2017, 7, 60.
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human-Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870.
- Mellor, D.J.; Beausoleil, N.J. Extending the ‘Five Domains’ model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 2015, 24, 241–253.
