Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The shoot apical meristem (SAM) gives rise to the aerial structure of plants by producing lateral organs and other meristems. The SAM is responsible for plant developmental patterns, thus determining plant morphology and, consequently, many agronomic traits such as the number and size of fruits and flowers and kernel yield.
- shoot apical meristem (SAM)
- SAM conservation
- Sc-RNA-seq
- streptophyta
1. Introduction
Plants can maintain indeterminate postembryonic growth by reserving pools of stem cells. These reservoirs are confined to specialized regions called meristems, which are present in various plant structures. The apical meristems (AMs) on the tips of the germinating seedling are responsible for the plant’s primary growth or length. There are two types of AMs: the shoot apical meristem (SAM) and the root apical meristem (RAM), which are responsible for the growth of the aerial part and the root system of plants, respectively. Additionally, plants have lateral meristems, which are responsible for secondary growth that determines the thickness of the plant; intercalary meristems, responsible for growth at the base of the nodes and leaf blades, mainly in monocots; and buds, which usually contain a small mass of meristematic tissue in spermatophytes [1][2][3].
2. Evolutionary Origin of the Meristem
2.1. The Concept of Stem Cells
Plant stem cells are typically found in meristems, and their defining characteristics include being cells specialized in producing new cells and organs, self-maintenance, slow proliferation with an extended G1 (or G0) cell state, pluripotency (ability to differentiate into multiple cell types), and regenerative potential or the ability to repopulate after damage [4][5][6]. The study of apical meristems (AMs) is particularly intriguing due to their stem cell pool, also known as promeristems, located at the central part of the shoot and the root apex. A continuous differentiating cell flux occurs throughout plant development from stem cells to proliferating cells, finishing with differentiating cell states [7]. Two types of plant cell differentiation exist: terminal and non-terminal. Terminal differentiation implies that a cell can no longer change its fate, while non-terminal differentiation allows a cell to change its fate when exposed to the correct signals, such as normal ontogeny, wounding, or other physiological changes [8]. Cells undergoing non-terminal differentiation appear to retain a stem cell potential [3]. However, the specific conditions activating their differentiation into various cell types remain largely unknown [9]. Standard models for studying plant cell differentiation include embryos, protoplasts, callus, and meristems [9][10][11][12]. These models are valuable as they contain pluripotent or totipotent cells that ultimately give rise to different plant tissues and even regenerate whole plants. Hence, an interesting perspective to understand this phenomenon is to focus on the evolution of pluripotency by comparing the meristems of early and modern diverged land plants, pointing out the common factors of pluripotency through evolution.
2.2. SAM and RAM
Extensive research has been conducted to understand RAM evolution, but little is known about the SAM evolutionary process. Since both AMs are structures with similar characteristics, studying the RAM can be a starting point for understanding the SAM. Some common attributes are that both are reservoirs of stem cells, both are promeristems that give rise to five primary meristems (protoderm, ground meristem, procambium, pericycle, and calyptrogen in the root [8]), and they are first determined during embryogenesis [13]. Stem cells in the root apical meristem (RAM) are present and maintained in the quiescent center (QC). In contrast, in the shoot apical meristem (SAM), stem cells are contained in the central zone (CZ) and maintained by the organizing center (OC) [14]. The QC and OC are the signaling centers responsible for stem cell maintenance in both meristems [14] and have been proposed to be functionally equivalent [15][16].
Studies conducted on the model plant Arabidopsis thaliana suggest that the organization of the signaling components required for stem cell initiation and maintenance in the RAM and SAM are relatively conserved. Consequently, similar classes of genes have been co-opted as central regulators in both types of meristems [16][17]. However, despite these shared mechanisms, the hormonal function is inverse [18][19]. In the SAM, auxins trigger differentiation, whereas in the RAM, they maintain the stem cell niche and support cell proliferation [20]. On the other hand, in the SAM, cytokinin promotes tissue proliferation of differentiating cells, whereas it promotes cell differentiation in the RAM [21][22]. The OC of the SAM is the site of maximal cytokinin activity, whereas the auxin maximum is in the QC of the RAM [22] (Figure 1).
Fossil evidence suggests that the RAM evolved at least twice independently rather than having a single origin [23][24]. The first appearance occurred in the lycophytes clade, followed by a second evolutionary event, likely in the ancestor of euphyllophytes (vascular plant non-lycophytes) [25]. It has even been proposed that RAM independently evolved multiple times in lycophytes [26]. Moreover, the similarities between lycophyte and euphyllophyte roots, including indeterminate growth, apical meristem protected by a root cap, root hairs, and a stele covered by a specialized endodermal cell layer, are clear examples of convergent evolution [27][28]. Genomic analyses revealed that limited gene expansion occurred at the divergence between the lycophyte and euphyllophyte clades [29]. Consequently, all the similarities produced by convergent evolution did not require extra gene families, suggesting that the rewiring of existing genetic programs was sufficient to generate multiple independent emergences of the RAM [30].
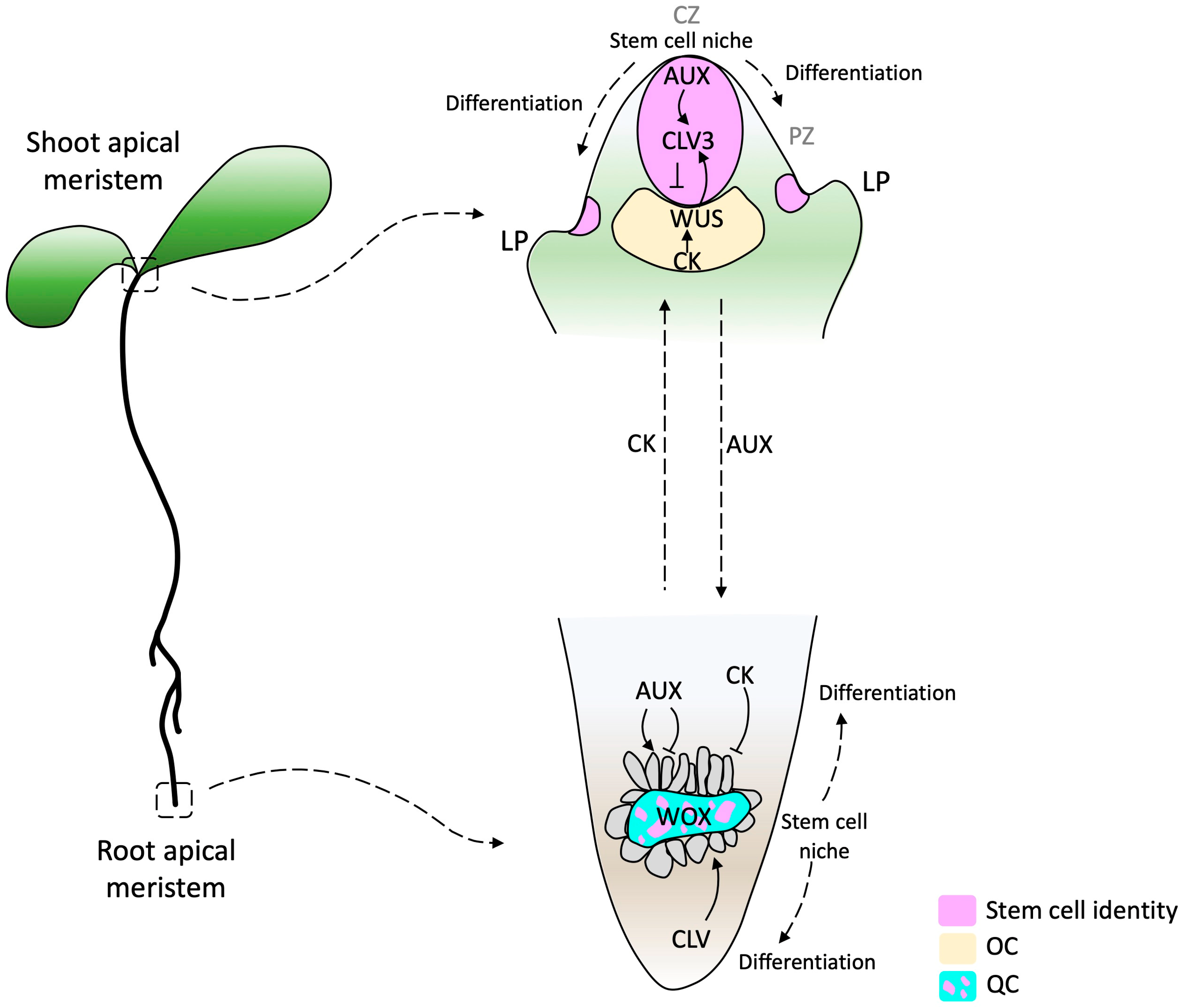
Figure 1. Diagram of SAM and RAM in Arabidopsis thaliana. In the SAM, a stem cell pool is located in the Central Zone (CZ, purple) above the organizing center (OC, yellow), which expresses the WUSCHEL transcription factor (TF) (yellow). Cells that pass the boundary defined by CLAVATA (CLV) function start differentiation, establishing the organ founder cell population. The RAM consists of a pool of quiescent cells (QC, cyan) surrounded by a pool of initial cells (the consideration of initial cells as stem cells depends on the author). This regulatory model highlights the complex interplay of phytohormones and TFs in the WOX domain. Arrows indicate activation and barred lines indicate inhibition. The dashed arrows beside auxin (AUX) and cytokinin (CK) indicate the hormone flow direction. AUX produced in the SAM and young leaves are basipetally transported through the stem by the polar auxin transport (PAT) stream toward the RAM. CK biosynthesis genes are expressed in the RAM differentiation zone and acropetally transported. CZ, central zone; PZ, peripheral zone; LP, lateral primordium [31][32].
2.3. Apical Meristem of Gametophyte and Sporophyte
The plant life cycle comprises two alternating phases: the haploid or gametophyte phase and the diploid or sporophyte phase. The diploid phase concludes with meiosis, while the haploid phase ends with the generation and union of gametes [33]. It is remarkable that, as land plants diversified, life cycle generation dominance twisted, and the sporophyte generation became dominant and increasingly specialized, whereas the gametophyte generation became reduced [34][35]. This trend is reflected in SAMs. Non-seed plants have simple meristems, sometimes with a single apical cell, to sustain their growth. In contrast, seed plants’ sporophytes develop complex multicellular meristems to support their growth and specialization, while their gametophytes lack meristems [36][37] (Figure 2). As both phases have meristems, it is reasonable to discuss the origin of meristems in terms of the plant life cycle.
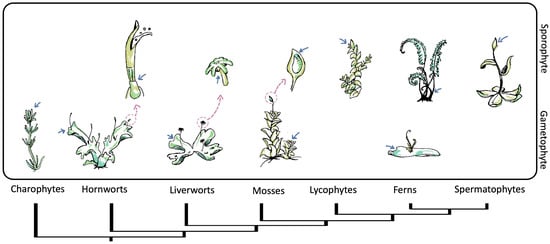
Figure 2. Phylogeny of streptophytes and schematic representation of the shoot apical meristem (SAM) location throughout gametophytic and sporophytic life stages. The pink arrows point to the position of the meristems, while the blue arrows indicate a close-up of specific meristematic structures. Within bryophytes and charophytes, the gametophyte is the dominant life state with vegetative growth from SAMs. Meanwhile in vascular plants, vegetative growth from SAMs occurs in a sporophyte state. The gametophytes of lycophytes and spermatophytes are not presented.
Two hypotheses have been proposed that explain the appearance of the alternation cycle [38]. First, the homologous hypothesis describes the ancestor of land plants with an originally haplontic life cycle that gives rise to a sporophyte through delay in meiosis, resulting in the intercalation of a new diploid organism [35][39]. This assumption outlines the gametophytic SAM as the ancestor of the sporophytic SAM. The second hypothesis is the antithetic hypothesis, which proposes that sporophytic SAM is independent of gametophytic SAM. According to this hypothesis, the sporophytic SAM evolved de novo by intercalating a system between embryonic and reproductive growth [35][40]. Current evidence based on embryophytes and fossil records is more compatible with the antithetic hypothesis than the homologous hypothesis [41][42]. Hypotheses derived from the antithetic hypothesis include (1) the embryophyte SAM originated from the transient seta meristem (sporophyte) of a bryophyte, (2) the hornwort sporophyte meristem is the possible ancestor of the SAM, and (3) the SAM in vascular plants arose de novo from a bryophyte ancestor [43].
Overall, evidence suggests that gametophyte and sporophyte SAM have an independent origin. However, because plants have retained a common set of genetic components since they diverged from algae, they are likely to share homologous regulatory loops and mechanisms in regulating their SAM. In fact, moss and fern gametophyte SAMs share key transcription factors (TFs) with angiosperm sporophyte SAMs [44][45], which suggests the existence of fundamental mechanisms involved in stem cell regulation across land plants.
3. Shoot Apical Meristem Regulation and Maintenance
The phenomena of branch development along the primary axis of plants, known as apical dominance, have been studied since the 1930s [46][47][48]. Over nine decades of research, a general concept emerged: the phytohormone auxin produced by the shoot tip is transported in a basipetal fashion by the polar transport stream, which inhibits axillary bud outgrowth [46][49]. Although this concept has been observed in angiosperms and gymnosperms [2][50][51][52], it has not been validated in other growth systems as dichotomous branching.
Auxin was the first plant regulator explored in SAM activity, and much has been learned about its signaling pathways in different organs. However, particular attention has been given to its interaction with cytokinin, another plant hormone. Auxins and cytokinins act synergistically or antagonistically to control SAM organization, formation, and maintenance [53]. For example, in Arabidopsis, cytokinins promote cell expansion, increasing SAM size, while auxin indirectly promotes differentiation through multiple mechanisms [54]. Despite being the most extensively studied regulators of plant development across plants, our understanding of the crosstalk between these hormones is still limited to a few plant models.
The apical dominance mechanism explains the classical observations; however, in addition to auxins and cytokinins, strigolactones (SLs) constitute a new class of phytohormones related to this mechanism. SLs were not considered in the classic literature because they were not introduced until the 2000s [55][56] and were soon linked to multiple developmental processes, such as shoot development [57][58][59][60]. Specifically, SLs play a crucial role in repressing bud outgrowth in monocots and in responses to environmental factors [58][61][62]. Recent studies suggest that the inhibition of bud outgrowth in apical dominance is attributed to the modulation of apically derived auxin flux by cytokinin and SLs [57][58].
3.1. The Regulatory Model: Angiosperms
With advances in molecular biology, SAM research has expanded from physiological aspects to the regulatory molecular mechanism. We understand that phytohormones and TFs cooperate to balance meristem maintenance and organ production. The canonical model of SAM regulation is based on the plant model Arabidopsis thaliana, and stands around the key genes CLAVATA (CLV, encoding ligand peptides) and WUSCHEL (WUS, encoding a homeobox TF) [63]. This regulatory loop also involves SHOOT MERISTEMLESS (STM), a KNOTTED1-LIKE HOMEOBOX (KNOX) TF [64][65]. The CLV gene family comprises the CLV peptides CLV3, CLV2, and CLV1. In this regulatory loop, WUS promotes stem cell identity and is regulated by CVL and STM. In addition, CLV favors organ initiation, and STM prevents the incorporation of central meristem cells into organ primordia [63][66] (Figure 3).
Other genes, such as the plant-specific GRAS TFs and HAIRY MERISTEM (HAM1–HAM4), interact with WUS/WOX5 (another member of the WUS family) [67]. A HAM concentration gradient modulates the WUS–CLVs interaction, promoting zonation of the SAM. In addition, members of the NAC group of leucine-rich TFs, including CUP-SHAPED COTYLEDON1 (CUC1), CUC2, and CUC3 [68], repeat receptor-like kinase genes (LRR-RLK), REVOLUTA (REV, a homeodomain TF), and the APETALA 2 (AP2) TF family, have been shown to have critical functions in the SAM [69][70].
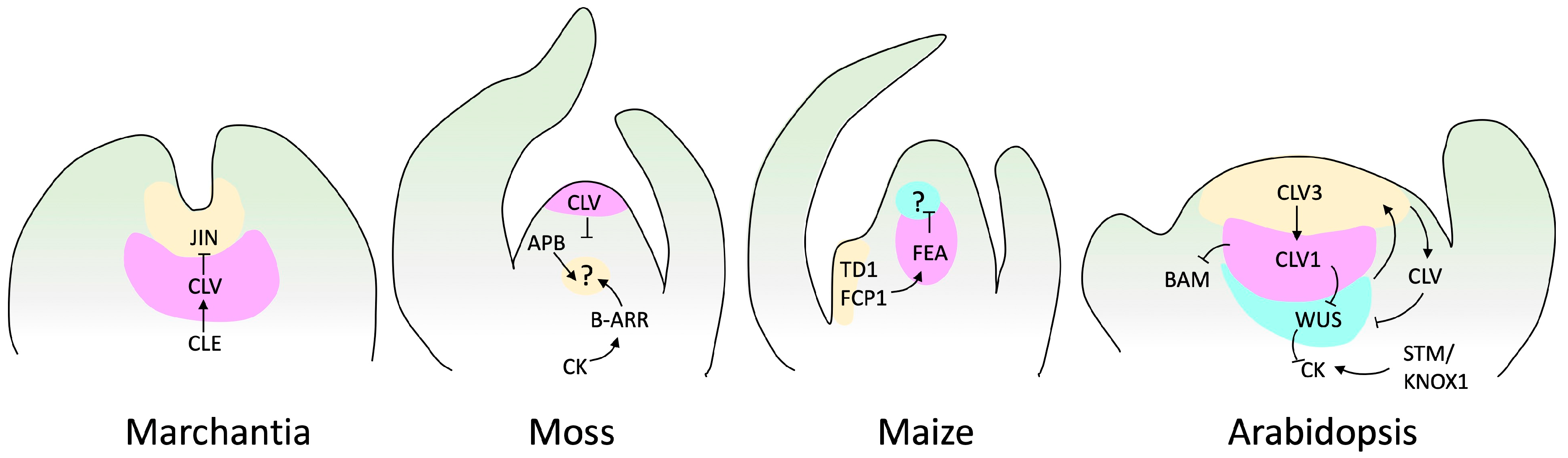
Figure 3. SAM signaling pathways in various species. This figure displays genes and factors, such as cytokinin (CK), with established genetic and biochemical interactions. Arrows indicate positive regulation, while barred lines represent negative regulation. Question marks denote unidentified receptors. Marchantia does not have a well-described loop to maintain meristem regulation. However, it has been reported that JINGASA (JIN) acts downstream of CLV3/ESR-related (CLE) peptide signaling and controls stem cell behavior in the gametophyte [71]. The structure of the moss meristem was originally reported by Hata and Kyozuka [36], and those of maize and Arabidopsis by Fletcher [72].
3.2. SAM TFs Conserved throughout Evolution
3.2.1. KNOX TFs
The KNOX genes belong to a large family of TFs called homeobox. They are involved in morphogenesis in all major eukaryotic lineages. In plants, members such as KN1, STM, and KNAT are directly associated with SAM maintenance [73]. Conservation of the KNOX gene family can be traced back to the plant’s ancestors, including Chlorophytes and Charophycean algae [73]. It has been suggested that KNOX transcription factors are part of the genetic toolkit that enabled the development of multicellularity, cell differentiation, and increased SAM complexity in seed plants [74]. Their functional roles are diverse across the phylogeny, including activation of the diploid phase, sporophyte and spore formation, meristem maintenance, and organogenesis [75][76]. In organisms such as Chlamydomonas reinhardtii, KNOX, and BELL TFs are inherited by gametes of the opposite mating types and heterodimerize in zygotes to activate diploid development [76][77]. Similarly, in Marchantia polymorpha, the expression in the gametophyte of KNOX and BELL is required to initiate zygotic development, while in Physcomitrella patens, KNOX expression is associated with sporophyte development and meristem regulation rather than the gametophyte [78]. In Lycophytes such as Sellaginella and spermatophytes, KNOX genes have been associated with cell proliferation and meristem maintenance [79][80]. Unfortunately, until now, there has not been a clear pattern of KNOX function across the phylogeny. However, duplication events followed by a sub-functionalization within each lineage appear to explain the presence of paralogs specialized for several developmental functions [73].
3.2.2. MADS TFs
The MADS-box gene family encodes TFs with a DNA-binding MADS domain, which was named after the proteins MINICHROMOSOME MAINTENANCE 1, AGAMOUS, DEFICIENS, and SERUM RESPONSE FACTOR (SRF). They are classified into two major classes based on their structure and phylogeny, Type I and Type II [81]. Type II classic MADS-box genes have been particularly well studied, as many have roles in determining floral organs. Type II MADS-box genes further diverged into two groups: MIKCC and MIKC* a [82]. Phylogenetic analyses suggest that algae MIKCC MADS-box genes could be considered the ancestral MIKCC before their divergence into the MIKC* and MIKCc clades. In Charophycean algae, MIKCC genes play a role in gamete differentiation [83].
In non-seed plants, MIKCCs have roles in gametophytic and sporophytic generations and contribute to the development of vegetative and reproductive structures [84][85][86]. However, in seed plants, their function is primarily linked to sporophyte development and the determination of floral organs [87][88]. On the other hand, MIKC* genes of non-seed and seed plants have a conserved role during gametophyte development [86][89]. It has been hypothesized that the function of MADS-box genes became restricted to specific plant organs after duplication events coinciding with the diversification of seed plants. Before that, MADS-box genes had multiple roles throughout plant development [90].
3.2.3. AP2/ERF TFs
The plant-specific APETALA 2/ethylene-responsive factor (AP2/ERF) family is characterized by the AP2 DNA-binding domain [91]. AP2/ERF genes are divided into classes based on the number of AP2 domains present. ERF-like genes contain one AP2 domain, while AP2-like genes contain two AP2 domains [92]. AP2-like genes can be further divided into the clades euANT, basalANT, and euAP2 according to the amino acid sequence of the double AP2 domain and the nuclear localization sequence [93]. In the model plant Arabidopsis thaliana and other angiosperms, all AP2-like clades play key roles in developmental processes and SAM regulation [74][94][95].
The AP2/ERF family is involved in diverse roles through plant evolution, although there is no experimental evidence of AP2-like function and no ANT sequences in algae. Phylogenetic analysis showed that an AP2 domain (AP2-R1 AA insertion) of the microalgae C. reinhardtii and Chlorokybus atmophyticus form a sister clade to the major clade of the AP2-like sequences of plants. However, they are distinct from euANTgene sequences, forming their own clade [96]. This suggests that C. reinhardtii and C. atmophyticus AP2 sequences could represent the putative ancestral sequence of the ANT group. In contrast, the AP2-like genes of multicellular algae, such as Mesotaenium caldariorum and Klebsormidium nitens, form a clade with land plants. It has been suggested that the ancestor of embryophytes may have a preANT-like gene which gave rise to the land plant exclusive basalANT and euANT lineages [92][95]. However, the diverse functions of this gene family increased following the plant’s evolutionary novelties; for example, in ferns, the expression of ANT has been reported in young sporangia, gametes, and spores, in gymnosperms in the ovule and during seed development. In angiosperms, they are involved in the meristem, flower organ, and fruit development [95][97][98].
This entry is adapted from the peer-reviewed paper 10.3390/ijms25031519
References
- Murray, J.A.H.; Jones, A.; Godin, C.; Traas, J. Systems Analysis of Shoot Apical Meristem Growth and Development: Integrating Hormonal and Mechanical Signaling. Plant Cell 2012, 24, 3907–3919.
- Kozlowski, T.T.; Theodore, T. Growth and Development of Trees; Academic Press: New York, NY, USA, 1971; ISBN 9780124242012.
- Jacobs, W.P. The Development of the Gynophore of the Peanut Plant, Arachis hypogaea L. I. The Distribution of Mitoses, the Region of Greatest Elongation, and the Maintenance of Vascular Continuity in the Intercalary Meristem. Am. J. Bot. 1947, 34, 361–370.
- Ivanov, V.B. The Problem of Stem Cells in Plants. Russ. J. Dev. Biol. 2023, 34, 205–212.
- Barlow, P.W. Stem Cells and Tissue Homeostasis; Cambridge University Press: Cambridge, UK, 1978; ISBN 9780521217996.
- Clark, S.E. Organ Formation at the Vegetative Shoot Meristem. Plant Cell 1997, 9, 1067–1076.
- Dubrovsky, J.G.; Ivanov, V.B. The Quiescent Centre of the Root Apical Meristem: Conceptual Developments from Clowes to Modern Times. J. Exp. Bot. 2021, 72, 6687–6707.
- Lev-Yadun, S. Stem cells in plants are differentiated too. Curr. Top. Plant Biol. 2023, 4, 93–102.
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 442509.
- Boutilier, K.; Offringa, R.; Sharma, V.K.; Kieft, H.; Ouellet, T.; Zhang, L.; Hattori, J.; Liu, C.M.; Van Lammeren, A.A.M.; Miki, B.L.A.; et al. Ectopic Expression of BABY BOOM Triggers a Conversion from Vegetative to Embryonic Growth. Plant Cell 2002, 14, 1737–1749.
- Xu, M.; Du, Q.; Tian, C.; Wang, Y.; Jiao, Y. Stochastic Gene Expression Drives Mesophyll Protoplast Regeneration. Sci. Adv. 2021, 7, 8466–8477.
- Shim, S.; Kim, H.K.; Bae, S.H.; Lee, H.; Lee, H.J.; Jung, Y.J.; Seo, P.J. Transcriptome Comparison between Pluripotent and Non-Pluripotent Calli Derived from Mature Rice Seeds. Sci. Rep. 2020, 10, 21257.
- Stahl, Y.; Simon, R. Plant Primary Meristems: Shared Functions and Regulatory Mechanisms. Curr. Opin. Plant Biol. 2010, 13, 53–58.
- Seago, J.L.; Fernando, D.D. Anatomical Aspects of Angiosperm Root Evolution. Ann. Bot. 2013, 112, 223–238.
- Haecker, A.; Groß-Hardt, R.; Geiges, B.; Sarkar, A.; Breuninger, H.; Herrmann, M.; Laux, T. Expression Dynamics of WOX Genes Mark Cell Fate Decisions during Early Embryonic Patterning in Arabidopsis Thaliana. Development 2004, 131, 657–668.
- Bennett, T.; Scheres, B. Root Development-Two Meristems for the Price of One? Curr. Top. Dev. Biol. 2010, 91, 67–102.
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved Factors Regulate Signalling in Arabidopsis Thaliana Shoot and Root Stem Cell Organizers. Nature 2007, 446, 811–814.
- Skylar, A.; Wu, X. Regulation of Meristem Size by Cytokinin SignalingF. J. Integr. Plant Biol. 2011, 53, 446–454.
- Greb, T.; Lohmann, J.U. Plant Stem Cells. Curr. Biol. 2016, 26, R816–R821.
- Mähönen, A.P.; ten Tusscher, K.; Siligato, R.; Smetana, O.; Díaz-Triviño, S.; Salojärvi, J.; Wachsman, G.; Prasad, K.; Heidstra, R.; Scheres, B. PLETHORA Gradient Formation Mechanism Separates Auxin Responses. Nature 2014, 515, 125–129.
- Dello Ioio, R.; Galinha, C.; Fletcher, A.G.; Grigg, S.P.; Molnar, A.; Willemsen, V.; Scheres, B.; Sabatini, S.; Baulcombe, D.; Maini, P.K.; et al. A PHABULOSA/Cytokinin Feedback Loop Controls Root Growth in Arabidopsis. Curr. Biol. 2012, 22, 1699–1704.
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The Yin-Yang of Hormones: Cytokinin and Auxin Interactions in Plant Development. Plant Cell 2015, 27, 44–63.
- Friedman, W.E.; Moore, R.C.; Purugganan, M.D. The Evolution of Plant Development. Am. J. Bot. 2004, 91, 1726–1741.
- Hetherington, A.J.; Dolan, L. Stepwise and Independent Origins of Roots among Land Plants. Nature 2018, 561, 235–238.
- Liu, W.; Xu, L. Recruitment of IC-WOX Genes in Root Evolution. Trends Plant Sci. 2018, 23, 490–496.
- Fujinami, R.; Yamada, T.; Imaichi, R. Root Apical Meristem Diversity and the Origin of Roots: Insights from Extant Lycophytes. J. Plant Res. 2020, 133, 291–296.
- Huang, L.; Schiefelbein, J. Conserved Gene Expression Programs in Developing Roots from Diverse Plants. Plant Cell 2015, 27, 2119–2132.
- Augstein, F.; Carlsbecker, A. Getting to the Roots: A Developmental Genetic View of Root Anatomy and Function from Arabidopsis to Lycophytes. Front. Plant Sci. 2018, 9, 411407.
- Leebens-Mack, J.H.; Barker, M.S.; Carpenter, E.J.; Deyholos, M.K.; Gitzendanner, M.A.; Graham, S.W.; Grosse, I.; Li, Z.; Melkonian, M.; Mirarab, S.; et al. One Thousand Plant Transcriptomes and the Phylogenomics of Green Plants. Nature 2019, 574, 679–685.
- Ferrari, C.; Shivhare, D.; Hansen, B.O.; Pasha, A.; Esteban, E.; Provart, N.J.; Kragler, F.; Fernie, A.; Tohge, T.; Mutwil, M. Expression Atlas of Selaginella Moellendorffii Provides Insights into the Evolution of Vasculature, Secondary Metabolism, and Roots. Plant Cell 2020, 32, 853–870.
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin-Cytokinin Interaction Regulates Meristem Development. Mol. Plant 2011, 4, 616–625.
- Luo, L.; Zeng, J.; Wu, H.; Tian, Z.; Zhao, Z. A Molecular Framework for Auxin-Controlled Homeostasis of Shoot Stem Cells in Arabidopsis. Mol. Plant 2018, 11, 899–913.
- Allen, C.E. The American Society of Naturalists Haploid and Diploid Generations. Am. Nat. 1937, 71, 734.
- Albert, V.A. Shoot Apical Meristems and Floral Patterning: An Evolutionary Perspective. Trends Plant Sci. 1999, 4, 84–86.
- Ligrone, R.; Duckett, J.G.; Renzaglia, K.S. The Origin of the Sporophyte Shoot in Land Plants: A Bryological Perspective. Ann. Bot. 2012, 110, 935.
- Hata, Y.; Kyozuka, J. Fundamental Mechanisms of the Stem Cell Regulation in Land Plants: Lesson from Shoot Apical Cells in Bryophytes. Plant Mol. Biol. 2021, 107, 213–225.
- Wu, X.; Yan, A.; McAdam, S.A.M.; Banks, J.A.; Zhang, S.; Zhou, Y. Timing of Meristem Initiation and Maintenance Determines the Morphology of Fern Gametophytes. J. Exp. Bot. 2021, 72, 6990–7001.
- Haig, D. Homologous Versus Antithetic Alternation of Generations and the Origin of Sporophytes. Bot. Rev. 2008, 74, 395–418.
- Niklas, K.J.; Kutschera, U. The Evolution of the Land Plant Life Cycle. New Phytol. 2010, 185, 27–41.
- Bennici, A. Origin and Early Evolution of Land Plants Problems and Considerations. Commun. Integr. Biol. 2008, 1, 212–218.
- Hemsley, A.R. The origin of the land plant sporophyte: An interpolational scenario. Biol. Rev. 1994, 69, 263–273.
- Kenrick, P. Alternation of generations in land plants: New phylogenetic and palaeobotanical evidence. Biol. Rev. 1994, 69, 293–330.
- Harrison, C.J. Auxin Transport in the Evolution of Branching Forms. New Phytol. 2017, 215, 545–551.
- Frank, M.H.; Scanlon, M.J. Transcriptomic Evidence for the Evolution of Shoot Meristem Function in Sporophyte-Dominant Land Plants through Concerted Selection of Ancestral Gametophytic and Sporophytic Genetic Programs. Mol. Biol. Evol. 2015, 32, 355–367.
- Youngstrom, C.E.; Geadelmann, L.F.; Irish, E.E.; Cheng, C.L. A Fern WUSCHEL-RELATED HOMEOBOX Gene Functions in Both Gametophyte and Sporophyte Generations. BMC Plant Biol 2019, 19, 416.
- Kebrom, T.H. A Growing Stem Inhibits Bud Outgrowth—The Overlooked Theory of Apical Dominance. Front. Plant Sci. 2017, 8, 309506.
- Thimann, K.V. Auxins and the Inhibition of Plant Growth. Biol. Rev. 1939, 14, 314–337.
- Went, F.W. Auxin, the Plant Growth-Hormone. Review 1935, 1, 162–182.
- Sussex, I.M.; Kerk, N.M. The Evolution of Plant Architecture. Curr. Opin. Plant Biol. 2001, 4, 33–37.
- Cline, M.G. Execution of the Auxin Replacement Apical Dominance Experiment in Temperate Woody Species. Am. J. Bot. 2000, 87, 182–190.
- Norstog, K.; Nicholls, T.J. The Biology of the Cycads, 1st ed.; Cornell University Press: New York, NY, USA, 1998.
- Napoli, C.A.; Beveridge, C.A.; Snowden, K.C. 5 Reevaluating Concepts of Apical Dominance and the Control of Axillary Bud Outgrowth. Curr. Top. Dev. Biol. 1998, 44, 127–169.
- Zhao, Y. Auxin Biosynthesis and Its Role in Plant Development. Annu. Rev. Plant Biol. 2010, 61, 49–64.
- Azizi, P.; Rafii, M.Y.; Maziah, M.; Abdullah, S.N.A.; Hanafi, M.M.; Latif, M.A.; Rashid, A.A.; Sahebi, M. Understanding the Shoot Apical Meristem Regulation: A Study of the Phytohormones, Auxin and Cytokinin, in Rice. Mech. Dev. 2015, 135, 1–15.
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone Inhibition of Shoot Branching. Nature 2008, 455, 189–194.
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of Shoot Branching by New Terpenoid Plant Hormones. Nature 2008, 455, 195–200.
- Bertheloot, J.; Barbier, F.; Boudon, F.; Perez-Garcia, M.D.; Péron, T.; Citerne, S.; Dun, E.; Beveridge, C.; Godin, C.; Sakr, S. Sugar Availability Suppresses the Auxin-Induced Strigolactone Pathway to Promote Bud Outgrowth. New Phytol. 2020, 225, 866–879.
- Zhang, J.; Mazur, E.; Balla, J.; Gallei, M.; Kalousek, P.; Medveďová, Z.; Li, Y.; Wang, Y.; Prát, T.; Vasileva, M.; et al. Strigolactones Inhibit Auxin Feedback on PIN-Dependent Auxin Transport Canalization. Nat. Commun. 2020, 11, 3508.
- Xie, Y.; Liu, Y.; Ma, M.; Zhou, Q.; Zhao, Y.; Zhao, B.; Wang, B.; Wei, H.; Wang, H. Arabidopsis FHY3 and FAR1 Integrate Light and Strigolactone Signaling to Regulate Branching. Nat. Commun. 2020, 11, 1955.
- Wu, F.; Gao, Y.; Yang, W.; Sui, N.; Zhu, J. Biological Functions of Strigolactones and Their Crosstalk With Other Phytohormones. Front. Plant Sci. 2022, 13, 821563.
- López-Ráez, J.A.; Charnikhova, T.; Gómez-Roldán, V.; Matusova, R.; Kohlen, W.; De Vos, R.; Verstappen, F.; Puech-Pages, V.; Bécard, G.; Mulder, P.; et al. Tomato Strigolactones Are Derived from Carotenoids and Their Biosynthesis Is Promoted by Phosphate Starvation. New Phytol. 2008, 178, 863–874.
- Shinohara, N.; Taylor, C.; Leyser, O. Strigolactone Can Promote or Inhibit Shoot Branching by Triggering Rapid Depletion of the Auxin Efflux Protein PIN1 from the Plasma Membrane. PLoS Biol. 2013, 11, e1001474.
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.X.; Jürgens, G.; Laux, T. The Stem Cell Population of Arabidopsis Shoot Meristems Is Maintained by a Regulatory Loop between the CLAVATA and WUSCHEL Genes. Cell 2000, 100, 635–644.
- Laux, T.; Mayer, K.F.X.; Berger, J.; Jürgens, G. The WUSCHEL Gene Is Required for Shoot and Floral Meristem Integrity in Arabidopsis. Development 1996, 122, 87–96.
- Long, J.A.; Moan, E.I.; Medford, J.I.; Barton, M.K. A Member of the KNOTTED Class of Homeodomain Proteins Encoded by the STM Gene of Arabidopsis. Nature 1996, 379, 66–69.
- Endrizzi, K.; Moussian, B.; Haecker, A.; Levin, J.Z.; Laux, T. The SHOOT MERISTEMLESS Gene Is Required for Maintenance of Undifferentiated Cells in Arabidopsis Shoot and Floral Meristems and Acts at a Different Regulatory Level than the Meristem Genes WUSCHEL and ZWILLE. Plant J. 1996, 10, 967–979.
- Zhou, X.; Guo, Y.; Zhao, P.; Sun, M.X. Comparative Analysis of WUSCHEL-Related Homeobox Genes Revealed Their Parent-of-Origin and Cell Type-Specific Expression Pattern during Early Embryogenesis in Tobacco. Front. Plant Sci. 2018, 9, 337885.
- Aida, M.; Tasaka, M. Genetic Control of Shoot Organ Boundaries. Curr. Opin. Plant Biol. 2006, 9, 72–77.
- Zhang, W. Putting Genes on the Map: Spatial Transcriptomics of the Maize Shoot Apical Meristem. Plant Physiol. 2022, 188, 1931–1932.
- Shimotohno, A.; Scheres, B. Topology of Regulatory Networks That Guide Plant Meristem Activity: Similarities and Differences. Curr. Opin. Plant Biol. 2019, 51, 74–80.
- Takahashi, G.; Kiyosue, T.; Hirakawa, Y. Control of Stem Cell Behavior by CLE-JINGASA Signaling in the Shoot Apical Meristem in Marchantia polymorpha. bioRxiv 2023.
- Fletcher, J.C. The CLV-WUS Stem Cell Signaling Pathway: A Roadmap to Crop Yield Optimization. Plants 2018, 7, 87.
- Graham, L.E.; Cook, M.E.; Busse, J.S. The Origin of Plants: Body Plan Changes Contributing to a Major Evolutionary Radiation. Proc. Natl. Acad. Sci. USA 2000, 97, 4535–4540.
- Floyd, S.K.; Bowman, J.L. The Ancestral Developmental Tool Kit of Land Plants. Int. J. Plant Sci. 2007, 168, 1–35.
- Dierschke, T.; Flores-Sandoval, E.; Rast-Somssich, M.I.; Althoff, F.; Zachgo, S.; Bowman, J.L. Gamete Expression of Tale Class Hd Genes Activates the Diploid Sporophyte Program in Marchantia polymorpha. Elife 2021, 10, e57088.
- Hisanaga, T.; Fujimoto, S.; Cui, Y.; Sato, K.; Sano, R.; Yamaoka, S.; Kohchi, T.; Berger, F.; Nakajima, K. Deep Evolutionary Origin of Gamete-Directed Zygote Activation by KNOX/BELL Transcription Factors in Green Plants. Elife 2021, 10, e57090.
- Lee, J.H.; Lin, H.; Joo, S.; Goodenough, U. Early Sexual Origins of Homeoprotein Heterodimerization and Evolution of the Plant KNOX/BELL Family. Cell 2008, 133, 829–840.
- Sakakibara, K.; Nishiyama, T.; Deguchi, H.; Hasebe, M. Class 1 KNOX Genes Are Not Involved in Shoot Development in the Moss Physcomitrella Patens but Do Function in Sporophyte Development. Evol. Dev. 2008, 10, 555–566.
- Harrison, C.J.; Coriey, S.B.; Moylan, E.C.; Alexander, D.L.; Scotland, R.W.; Langdale, J.A. Independent Recruitment of a Conserved Developmental Mechanism during Leaf Evolution. Nature 2005, 434, 509–514.
- Kawai, J.; Tanabe, Y.; Soma, S.; Ito, M. Class 1 KNOX Gene Expression Supports the Selaginella Rhizophore Concept. J. Plant Biol. 2010, 53, 268–274.
- Alvarez-Buylla, E.R.; Liljegren, S.J.; Pelaz, S.; Gold, S.E.; Burgeff, C.; Ditta, G.S.; Vergara-Silva, F.; Yanofsky, M.F. MADS-Box Gene Evolution beyond Flowers: Expression in Pollen, Endosperm, Guard Cells, Roots and Trichomes. Plant J. 2000, 24, 457–466.
- Henschel, K.; Kofuji, R.; Hasebe, M.; Saedler, H.; Münster, T.; Theißen, G. Two Ancient Classes of MIKC-Type MADS-Box Genes Are Present in the Moss Physcomitrella Patens. Mol. Biol. Evol. 2002, 19, 801–814.
- Tanabe, Y.; Hasebe, M.; Sekimoto, H.; Nishiyama, T.; Kitani, M.; Henschel, K.; Münster, T.; Theissen, G.; Nozaki, H.; Ito, M. Characterization of MADS-Box Genes in Charophycean Green Algae and Its Implication for the Evolution of MADS-Box Genes. Proc. Natl. Acad. Sci. USA 2005, 102, 2436–2441.
- Singer, S.D.; Krogan, N.T.; Ashton, N.W. Clues about the Ancestral Roles of Plant MADS-Box Genes from a Functional Analysis of Moss Homologues. Plant Cell Rep. 2007, 26, 1155–1169.
- Koshimizu, S.; Kofuji, R.; Sasaki-Sekimoto, Y.; Kikkawa, M.; Shimojima, M.; Ohta, H.; Shigenobu, S.; Kabeya, Y.; Hiwatashi, Y.; Tamada, Y.; et al. Physcomitrella MADS-Box Genes Regulate Water Supply and Sperm Movement for Fertilization. Nat. Plants 2018, 4, 36–45.
- Thangavel, G.; Nayar, S. A Survey of MIKC Type MADS-Box Genes in Non-Seed Plants: Algae, Bryophytes, Lycophytes and Ferns. Front. Plant Sci. 2018, 9, 342764.
- Becker, A.; Theißen, G. The Major Clades of MADS-Box Genes and Their Role in the Development and Evolution of Flowering Plants. Mol. Phylogenet. Evol. 2003, 29, 464–489.
- Ferrario, S.; Shchennikova, A.V.; Franken, J.; Immink, R.G.H.; Angenent, G.C. Control of Floral Meristem Determinacy in Petunia by MADS-Box Transcription Factors. Plant Physiol. 2006, 140, 890–898.
- Zobell, O.; Faigl, W.; Saedler, H.; Münster, T. MIKC* MADS-Box Proteins: Conserved Regulators of the Gametophytic Generation of Land Plants. Mol. Biol. Evol. 2010, 27, 1201–1211.
- Ambrose, B.A.; Smalls, T.L.; Zumajo-Cardona, C. All Type II Classic MADS-Box Genes in the Lycophyte Selaginella Moellendorffii Are Broadly yet Discretely Expressed in Vegetative and Reproductive Tissues. Evol. Dev. 2021, 23, 215–230.
- Riechmann, J.L.; Meyerowitz, E.M. The AP2/EREBP Family of Plant Transcription Factors. Biol. Chem. 1998, 379, 633–654.
- Gutterson, N.; Reuber, T.L. Regulation of Disease Resistance Pathways by AP2/ERF Transcription Factors. Curr. Opin. Plant Biol. 2004, 7, 465–471.
- Zhao, Y.; Ma, R.; Xu, D.; Bi, H.; Xia, Z.; Peng, H. Genome-Wide Identification and Analysis of the AP2 Transcription Factor Gene Family in Wheat (Triticum Aestivum L.). Front. Plant Sci. 2019, 10, 486684.
- Kim, S.; Soltis, P.S.; Wall, K.; Soltis, D.E. Phylogeny and Domain Evolution in the APETALA2-like Gene Family. Mol. Biol. Evol. 2006, 23, 107–120.
- Zumajo-Cardona, C.; Vasco, A.; Ambrose, B.A. The Evolution of the KANADI Gene Family and Leaf Development in Lycophytes and Ferns. Plants 2019, 8, 313.
- Dipp-álvarez, M.; Cruz-Ramírez, A. A Phylogenetic Study of the ANT Family Points to a PreANT Gene as the Ancestor of Basal and EuANT Transcription Factors in Land Plants. Front. Plant Sci. 2019, 10, 17.
- Bui, L.T.; Pandzic, D.; Youngstrom, C.E.; Wallace, S.; Irish, E.E.; Szövényi, P.; Cheng, C.L. A Fern AINTEGUMENTA Gene Mirrors BABY BOOM in Promoting Apogamy in Ceratopteris richardii. Plant J. 2017, 90, 122–132.
- Krizek, B.A.; Bantle, A.T.; Heflin, J.M.; Han, H.; Freese, N.H.; Loraine, A.E. AINTEGUMENTA and AINTEGUMENTA-LIKE6 Directly Regulate Floral Homeotic, Growth, and Vascular Development Genes in Young Arabidopsis Flowers. J. Exp. Bot. 2021, 72, 5478–5493.
This entry is offline, you can click here to edit this entry!
