Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Respiratory System
Chemerin is an atypical chemokine first described as a chemoattractant agent for monocytes, natural killer cells, plasmacytoid and myeloid dendritic cells, through interaction with its main receptor, the G protein-coupled receptor chemokine-like receptor 1 (CMKLR1). Chemerin has been studied in various lung disease models, showing both pro- and anti-inflammatory properties. Given the incidence and burden of inflammatory lung diseases from diverse origins (infectious, autoimmune, age-related, etc.), chemerin has emerged as an interesting therapeutical target due to its immunomodulatory role.
- chemerin
- RARRES2
- CMKLR1
- lung
- inflammation
1. Chemerin and Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS) is an acute inflammation of the lung linked to a sudden increase in alveolocapillary permeability, leading to decreased lung compliance, respiratory dysfunction and hypoxemia [29]. The diagnosis of ARDS is based on the Berlin criteria: 1. Development of an acute hypoxemia within 1 week of a known injury or new onset or worsening of respiratory symptoms. 2. Bilateral lung opacities on chest imaging. 3. Absence of a cardiac cause [30]. The severity of arterial hypoxemia determines the severity of ARDS in mild (PaO2/FIO2 ≤ 300 mm Hg), moderate (PaO2/FIO2 ≤ 200 mm Hg) and severe (PaO2/FIO2 ≤ 100 mm Hg). ARDS is responsible for 10% of intensive care unit admissions, and mortality approaches 50% in severe cases [31]. Etiologies are multiple, including either direct damage to the lung, mostly by viral or bacterial pneumonia, or an indirect lesion, notably secondary to sepsis or a severe trauma.
The most commonly used mouse model of ARDS is the acute lung injury (ALI) model that consists in the administration of bacterial lipopolysaccharide (LPS) [32]. This leads to a massive infiltration of neutrophils which peaks after 24 h, a development of edema and an intra-alveolar hemorrhage [33,34]. This model is considered as a model of mild ARDS [30]. Researchers have previously shown in the direct ALI model induced by LPS that the addition of recombinant chemerin to LPS led to a decreased immune response characterized by a lower recruitment of neutrophils in the lungs and bronchoalveolar lavage fluid (BALF) and resulting in fewer histological lesions [35]. Pro-inflammatory cytokines were also decreased in the BALF of mice receiving recombinant chemerin and LPS. As expected, CMKLR1 knock-out (CMKLR1KO) mice did not respond to recombinant chemerin and presented higher levels of neutrophils in the lungs and BALF compared to WT mice after LPS challenge [35].
A recent study by Mannes et al. used an agonist of CMKLR1 coupled with 64Cu as a radiotracer to visualize the recruitment of CMKLR1-positive cells with positron emission tomography (PET). They observed a higher and significant uptake of the radiotracer in LPS-treated mice compared to control mice at days 1, 2, 4 and after LPS intratracheal instillation, with a maximal uptake at day 2. This increase was mostly linked to an uptake by monocytes-derived macrophages and to a lesser extent of interstitial macrophages and monocytes [36]. These results are compatible with the previously described chemoattractant role of chemerin in the LPS model of direct ALI.
In contrast to the results described above, Provoost et al. showed a pro-inflammatory role of the chemerin/CMKLR1 system in another model of acute lung inflammation induced by exposing the mice to diesel exhaust particles (DEP). They first observed that chemerin concentration in BALF was increased in WT mice exposed to DEP compared to control mice. This rise was associated with a decrease in the expression of chemerin in alveolar epithelial cells, suggesting a release of chemerin by these cells in the case of DEP exposure. CMKLR1KO mice exposed to DEP presented a lower recruitment of monocytes and dendritic cells, as well as reduced levels of pro-inflammatory cytokines [37].
Another model of direct ALI is with exposure to ozone (O3) [38]. Razvi et al. exposed wild-type mice to O3 for 3 h and collected BALF 24 h later. Higher chemerin concentrations were observed in O3-exposed mice compared to control mice [39]. This model was then applied to CCRL2KO mice and, although they presented the same degree of severity as WT mice, they had significantly higher chemerin concentrations in their BALF, confirming the role of CCRL2 as a decoy receptor [40]. These results indicate that CCRL2 has no evident role in the effects of chemerin during ARDS. Moreover, the fact that increased chemerin levels in the CCRL2KO mice did not change the responses to O3 exposure may indicate that the maximum dose effect of chemerin was already reached in the WT mice.
Zou et al. used a model of indirect ALI in rats induced by limb ischemia/reperfusion. They observed an increase in the levels of chemerin in the lungs of rats as compared to controls. Rats treated with hydrogen-saturated physiological saline solution, a fluid showing protective lung effects in the case of ischemia/reperfusion, presented a decrease in chemerin lung levels that was strongly correlated with a decreased lung injury score as evaluated histologically [41].
All these studies highlight that the chemerin/CMKLR1 system is associated with acute inflammation, with higher chemerin levels measured in rodents with ALI [37,39,41] and an infiltration of CMKLR1-positive cells in the latter phases of the LPS model [36]. However, models of direct ALI induced by either LPS or DEP showed contradictory results, with an anti-inflammatory role for the first model [35] and a pro-inflammatory one for the second [37]. Even if LPS and DEP both activate the innate immune system through interaction with toll-like receptor 4, the intensity of the immune response is different. Indeed, the total number of neutrophils in the BALF is more than twice as high in mice receiving LPS as mice exposed to DEP [35,37,42,43]. Intratracheal instillation of chemerin reduced the inflammation induced by LPS instillation, possibly favoring the recruitment of a cell population with anti-inflammatory properties [35].
2. Chemerin, Lung Infection and Sepsis
As the chemerin/CMKLR1 system is involved in the physiopathology of lung acute inflammation, it makes sense that it could also be involved in lung infection and sepsis. This text focused on severe lung pneumonia, a respiratory infection that affects the lower respiratory tract. It can be caused by various microorganisms, including viruses (e.g., influenza, SARS-CoV-2, RSV) and bacteria (e.g., streptococcus pneumoniae). Bacterial pneumonia is the deadliest infection, with a mortality rate approaching 20% among patients older than 85 years [44].
Chemerin levels were assessed in several sepsis and lung infection conditions. Regarding COVID-19 infection, one group reported higher serum chemerin concentrations in healthy controls compared to COVID-19 patients [45]. In a second study, the same group did not highlight significant differences in serum chemerin levels between non-severe and severe COVID-19 patients [46]. They observed a decrease in chemerin concentrations between days 1 and 7, followed by an increase between days 7 and 28 in moderate and severe patients, with a continuous increase in mild patients [46]. The authors did not find any correlation between chemerin levels and inflammatory biomarkers and hypothesized that the increase in serum chemerin concentrations was associated with the resolution of inflammation. However, chemerin levels observed in these two studies were significantly different, in the pg/mL range when measured with multiplex in Sulicka-Grodzicka et al. [46] and in the ng/mL range when assessed with ELISA in Kukla et al. [45], making it difficult to interpret their data (Figure 1). Esendagli et al. also measured the chemerin serum concentration of COVID-19 patients at hospital admission and separated them according to their prognosis, which depended on the type of ventilation and survival. Patients with a good prognosis presented higher chemerin levels at admission compared to patients with a bad prognosis; however, no significant difference was observed if patients had lung sequellae or not [47].
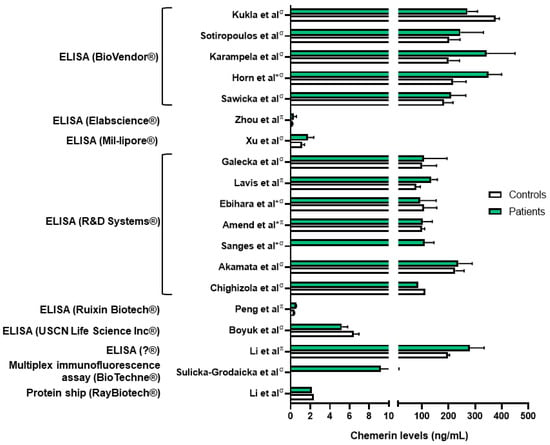
Figure 1. Chemerin levels measured in different studies from patients with various lung diseases (green column bars) and controls (white column bars). σ: measures performed in serum samples; π: measures performed in plasma samples; *: chemerin levels extrapolated from graphs. Data from [45,48,49,50,51,54,57,59,60,61,63,64,65,66,67,71,72,73]. Figure created with Biorender.com.
These results are contradictory to the ones obtained by others. Indeed, researchers observed higher chemerin plasmatic levels in COVID-19 patients compared to healthy controls, and patients hospitalized in the intensive care unit had the highest levels [54]. This is in agreement with previously described results showing a positive correlation between chemerin plasma concentration and inflammatory biomarkers [79]. Moreover, researchers demonstrated that chemerin concentrations on day 14 of hospitalization were an independent risk factor of mortality. The researchers also showed by the histological examination of lungs from autopsied COVID-19 patients that chemerin is mainly expressed by fibroblasts/myofibroblasts in fibrotic lesions of diffuse alveolar damage [54].
The results are in agreement with measurements of chemerin concentration obtained in septic patients. Indeed, Amend et al. observed higher chemerin plasma levels in COVID-19 patients compared to healthy controls. Of note, chemerin levels were identical between septic patients secondary to SARS-CoV-2 infection and other causes of sepsis, and no significant difference was observed between deceased or recovered patients. They also observed a positive and moderate correlation between chemerin levels and the C-reactive protein [48]. Concordant results were also obtained by Karampela et al. In this study, chemerin serum concentrations were higher at admission in septic patients compared to controls. The concentration was also higher in patients with septic shock, and chemerin levels could even discriminate patients with sepsis from patients with septic shock with a sensitivity and a specificity of approximately 70%. Chemerin levels were higher in deceased patients compared to recovered patients, and at days 1 and 7 of admission they were independent risk factors of mortality [49]. Similar conclusions were described by Horn et al., who observed higher chemerin levels in septic patients compared to controls, with higher levels in patients with a higher severity score for sepsis. This was also demonstrated in a mouse model of peritoneal septic shock, where mice with severe septic shock had the highest chemerin concentration [50]. Finally, one study did not observe any significant difference in serum chemerin levels between septic patients compared to controls, nor any difference according to mortality, but their cohort only included 37 septic patients and 12 controls [51].
Besides those observational studies, only a few studies have evaluated the role of the chemerin/CMKLR1 system in the pathophysiology of pneumonia. In a model of severe viral pneumonia induced by the pneumonia virus of mice, the mice counterpart of the respiratory syncytial virus (RSV), an anti-inflammatory role of the system was demonstrated. Indeed, CMKLR1KO mice presented a more severe disease with higher mortality, lung inflammation and viral titers. BALF chemerin levels were also increased. Anti-viral cytokines such as interferon-α were strongly decreased, while pro-inflammatory cytokines rose sharply. The increase in inflammatory cells was accompanied by a significant decrease in plasmacytoid dendritic cells (DC) and CD8+ T cells. Additional experiments suggested that the anti-inflammatory properties of the chemerin/CMKLR1 system depended on non-leucocytic cells [55]. In contrast, in vitro experiments showed that inactivation of RARRES2 decreased the sensibility of an alveolar basal epithelial cell line (A549 cells) to RSV infection, as observed by decreased viral replication [56]. The in vitro results could be explained by the lack of interference of the immune response to RSV infection. While chemerin may have a direct pro-viral role in lung cells, the anti-viral effects of this chemokine in the immune system may prevail, resulting in the beneficial effect observed in the in vivo model.
3. Chemerin and Obstructive Pulmonary Diseases
Asthma and chronic obstructive pulmonary disease (COPD) represent the most frequent chronic respiratory diseases, affecting around 7% of the population [80]. Both are obstructive pulmonary diseases, generally characterized by their reversible (asthma) or irreversible (COPD) nature. The immune response observed in asthmatic patients is mostly mediated by T helper 2 (Th2) cells which lead to an infiltration by eosinophils. Mast cells are also involved in the secretion of bronchoconstrictive mediators [81,82]. COPD develops as a result of repeated injuries to the airway epithelium driving a reprogramming of basal cells. The main immune cells involved in the pathogenesis of COPD are neutrophils and macrophages, but their precise roles are not well understood yet [83].
It has been previously shown that plasma chemerin concentrations were higher in patients with severe asthma compared to healthy controls [57]. A mouse model of allergic asthma induced by exposing the mice to ovalbumin highlighted the role of chemerin in the pathophysiology of this disease. Indeed, the addition of recombinant chemerin to ovalbumin led to a decrease in BALF immune cells, notably eosinophils, inflammatory DC and CD4+ T cells, along with a decrease in IL-4 and IL-13 BALF concentrations. The number of goblet cells was also reduced. The anti-inflammatory properties of chemerin were linked to a direct interaction with lung epithelial cells, leading to a decrease in CCL2 secretion, a chemoattractant agent for inflammatory DC, and not by a direct role on these immune cells [58]. Similar anti-inflammatory properties of chemerin were observed in a mouse model of aggravated allergic airway inflammation induced by simultaneously exposing mice to DEP and house dust mites. CMKLR1KO mice presented a higher recruitment of monocytes, neutrophils, eosinophils, DCs and T cells compared to WT mice [37].
Regarding the link between chemerin and COPD, an exhaustive review was recently published and only main results will be presented here [84]. Two independent studies reported higher chemerin levels in COPD patients compared to healthy controls [59,60]. While Li et al. observed a higher chemerin concentration in hospitalized COPD patients and a positive correlation between chemerin concentrations and number of hospitalizations over 6 months [60], Boyuk et al. could not show any difference in chemerin levels according to the severity of COPD [59]. Additionally, a third independent study did not demonstrate any difference in serum chemerin levels between COPD patients and matched healthy controls or any correlation with respiratory function [61].
In order to study the pathophysiological role of chemerin in COPD, WT and CMKLR1KO mice were exposed to tobacco smoke for 4 weeks (subacute exposure) or 24 weeks (chronic exposure). Both subacute and chronic exposure led to a decrease in preprochemerin and CMKLR1 expression in lungs from WT mice. However, only chronic exposure significantly decreased chemerin expression in the lung bronchial epithelium, whereas no effect for subacute exposure was observed. This decrease was associated with an increase in chemerin concentration in the BALF, suggesting, as previously described [37], a release of chemerin by epithelial cells. In this model, CMKLR1KO mice were partially protected against the subacute inflammatory response, with a lower recruitment of immune cells and reduced secretion of chemokines, showing a proinflammatory role for the chemerin/CMKLR1 axis [62].
4. Chemerin and Autoimmune Diseases
Autoimmune diseases are caused by deregulated responses of the immune system, leading to damage of self-tissue and disruption of its normal function.
Chemerin was mostly characterized in systemic sclerosis (SSc), an autoimmune connective disease, leading to fibrosis of the skin and internal organs [85]. Lungs can be affected during the course of the disease and their involvement is responsible for the death of most of SSc patients [86]. The main pulmonary lesions observed are interstitial lung disease (ILD) and pulmonary hypertension (PAH).
Measurements of chemerin serum concentration in patients with SSc gave contradictory results and were dependent on the population of SSc patients studied. Thus, either higher [63], no difference [64] and even lower chemerin concentrations [65] were observed in SSc patients as compared to controls. Recently, Sanges et al. compared SSc patients with or without PAH and observed higher levels of chemerin in serum from PAH-SSc patients. Chemerin levels in PAH-SSc patients were also higher compared to SSc patients with ILD but without PAH. They also observed an upregulation of the chemerin/CMKLR1 axis in the lung vessels of PAH-SSc patients compared to healthy controls [66]. On the other hand, a correlation between PAH and chemerin levels was not found by Sawicka et al., maybe due to their smaller patient cohort [63].
As chemerin and CMKLR1 are expressed by endothelial cells and CMKLR1 by smooth muscle cells, the link between the chemerin/CMKLR1 axis and PAH was investigated. In vitro studies showed that chemerin 9, the shortest chemerin-derived peptide that retains the highest potency against CMKLR1 [11], could induce a contraction of an isolated pulmonary artery and that the effect was greater when the arteries were isolated from rats with PAH. They also observed an increase in CMKLR1 and a decrease in CCRL2 expression in lungs from PAH rats as compared to control rats, as well as an increase in chemerin expression in plasma [69]. It was also demonstrated that recombinant chemerin potentiated the effects of vasoconstrictors such as phenylephedrine, endothelin 1 and serotonin and antagonized the vasodilatation effect of acetylcholine. In opposition to this study, it was demonstrated that recombinant chemerin led to pulmonary artery vascular contraction only in the absence of endothelium [70]. The same results were obtained by Peng et al., who observed an overexpression of chemerin and CMKLR1 in lungs from rats with PAH. Smooth muscle cells isolated from the pulmonary artery and treated with recombinant chemerin or exposed to hypoxia displayed a higher expression of chemerin and CMKLR1, suggesting an autocrine role for the system. Chemerin also favored the migration and proliferation of these cells. Moreover, the concentration of chemerin was significantly increased in plasma from idiopathic PAH patients, and a chemerin concentration above 471.76 pg/mL could predict a PAH diagnosis with a sensitivity of 85.7% and a specificity of 100% [67]. Explants from patients with idiopathic PAH were also analyzed and showed an upregulation of chemerin in fibroblasts from idiopathic PAH compared to controls [68].
Regarding rheumatoid arthritis, even if chemerin seems to be implicated in the physiopathology of the disease, no study has evaluated the link between chemerin and the pulmonary lesions associated to it [87].
5. Chemerin and Lung Cancer
The link between inflammation and cancer is a rapidly growing research area, and chemerin has been studied in cancer, and notably in lung cancer, as it was hypothesized that its chemoattractant properties could mediate the recruitment of tumor-associated immune cells and influence neoangiogenesis [88].
Zhao et al. found a seven-gene panel, including RARRES2, that was associated with the lung tumoral microenvironment. Patients with a higher expression of RARRES2 had a better overall survival. Using the seven-gene panel, they accurately divided patients into low risk and high risk of progression or death [76]. In line with this study, immunohistochemistry analysis of non-small cell lung carcinoma (NSCLC) revealed that chemerin expression was decreased in tumor cells compared to the normal adjacent stroma, and the more the tumor was differentiated, the more chemerin was expressed. Patients with higher chemerin expression had a better survival, and the expression of chemerin was demonstrated as an independent risk factor for five-year progression-free survival [74]. Similar results were obtained by Cai et al. [75]. Two independent teams assessed chemerin concentrations in serum from a large cohort of patients with NSCLC. They both observed higher serum chemerin levels in NSCLC patients and could discriminate NSCLC patients from healthy controls based on chemerin concentrations with a sensibility of around 63%. However, the cut-off of chemerin values differed strongly between the two cohorts, and contradictory results were obtained regarding the association between chemerin concentrations, lymph node involvement and tumoral stage. Of note, one cohort comprised only resectable NSCLC (absence of metastasis) and the other comprised around 30% of metastatic patients [71,72]. Only one smaller study did not find a significant difference in chemerin concentrations in serum in NSCLC patients compared to controls [73].
The mechanisms through which chemerin influences lung cancer have not been thoroughly investigated. However, certain in vivo models have attempted to unravel the connection between chemerin and the development of cancer. Mice inoculated with B16 melanoma tumor cells overexpressing chemerin had smaller tumors with an enhanced recruitment of NK and T cells [77]. It was also shown in the same disease model using CMKLR1KO and CCLR2KO mice that the decrease in the tumor size was mostly due to decreased tumor neoangiogenesis induced by chemerin, with no remarkable changes in lymphocyte infiltration [27]. Dubois-Vedrenne et al. observed in a model of chemical skin tumors that mice overexpressing a bioactive form of chemerin developed fewer and smaller tumors. These effects were partially mediated by CMKLR1 and were only linked to the action of chemerin in the latter stages of tumorigenesis [78]. Of note, the pro-tumoral effects of chemerin were only observed in in vitro models, with enhanced migration properties in models of squamous cell carcinoma [89,90]. In vitro models do not provide a comprehensive understanding of crucial cellular interactions, particularly those occurring between cancer cells and the tumor microenvironment. Given that the anti-tumoral effects of chemerin appear to be closely associated with responses from immune and endothelial cells, prioritizing in vivo studies becomes imperative.
This entry is adapted from the peer-reviewed paper 10.3390/cells13020171
This entry is offline, you can click here to edit this entry!
