In the 1940s and 1950s, the unrestrained use of oxygen (O
2) in industrialized nations sparked the initial epidemic of retinal disease in premature births, termed retrolental fibroplasia [
6,
7,
8,
9]. Subsequently, advancements in neonatal care and perinatal monitoring in the 1960s and 1970s increased the survival rate of extremely premature infants, causing a second surge in retinal disease [
10,
11]. This trend continued with a third peak affecting middle-income countries and regions like China, Southeast Asia, South Asia, South America, and Eastern Europe due to enhanced survival rates of very premature infants through improved neonatal care [
12,
13,
14,
15,
16,
17,
18,
19,
20]. More recently, European countries and the US have witnessed a rising trend in ROP incidence. For instance, a study in the UK revealed a 4% incidence of ROP requiring treatment among preterm babies weighing < 1500 g [
21]. Similarly, studies in Greece and Norway reported incidences of around 18.4% and 39.6%, respectively, among preterm infants [
22]. In the US, the incidence rose from 11% in 2009 to 15% in 2018 among neonates meeting ROP screening criteria [
23]. Currently, developed regions like the US and the UK tend to show a lower incidence, while developing regions such as India and Africa demonstrate slightly higher ROP incidence rates [
24].
2. Retinal Development and Disease Pathogenesis
During physiological retinal angiogenesis, blood vessels begin to form around the 14–15th week of gestation, originating from the optic nerve head and expanding centrifugally towards the retinal periphery [
82]. By 36 weeks of gestation, the nasal portion of the retina becomes vascularized, while the temporal area completes this process by the 40th week. Consequently, preterm infants exhibit incompletely vascularized retinas, with the extent of the avascular zone contingent upon their gestational age [
83].
Under normal circumstances, the hypoxic conditions typical of the intrauterine environment stimulate retinal vascularization by prompting the expression of hypoxia-inducible factor 1α (HIF-1α). This factor regulates the expression of various oxygen-sensing genes, including crucial proangiogenic factors like vascular endothelial growth factor (VEGF) [
84]. Although the VEGF family encompasses several members, including VEGF-A, -B, -C, -D, and placental growth factor (PlGF), it is VEGF-A that predominantly drives retinal angiogenesis [
85,
86].
VEGF, primarily released by neuroglia, initiates retinal blood vessel formation through the migration of vascular endothelial cells in a paracrine manner [
87]. As retinal angiogenesis progresses, hypoxic conditions diminish, leading to the cessation of HIF-1 activation and its target genes. However, premature exposure to oxygen (hyperoxia) in an immature retina significantly suppresses HIF-1 and VEGF activity, resulting in oxidative stress and the emergence of avascular retinal regions [
82]. Nitro-oxidative stress, characterized by an imbalance between the abundant generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and antioxidative defense mechanisms, leads to excess ROS and RNS. This imbalance triggers dramatic structural molecular changes and the activation of inflammatory and cell death pathways, a condition detectable in various eye diseases [
88,
89]. It is noteworthy that infants possess reduced antioxidant defenses [
90]. In this context, Buhimish and colleagues reported that preterm births exhibit a lack of compensatory upregulation of nonenzymatic antioxidant reserves, such as glutathione (GSH) and plasma total free radical-trapping antioxidant potential [
91]. Given the diminished antioxidative capability and increased vulnerability to oxidative stress in preterm neonates, it is not surprising that several oxidative biomarkers, such as malondialdehyde (MDA), 8-hydroxy 2-deoxyguanosine (8-OHdG), and the GSH/GSSG ratio, have been discussed as potential diagnostic tools for ROP [
92].
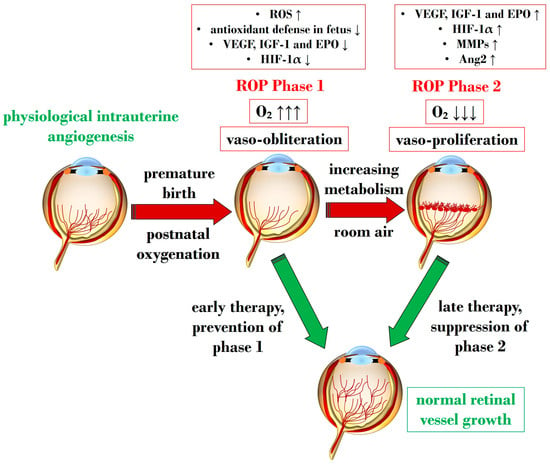
An overabundance of ROS is described during the first phase of ROP, a status of hyperoxia that manifests as vaso-obliteration, characterized by decreased levels of HIF-1α, VEGF, and IGF-1 [
93]. Subsequently, an ischemic phase ensues, gradually progressing into a proliferative stage marked by abnormal and dysfunctional neoangiogenesis. This phase ultimately results in intravitreal fibrosis, retinal traction, and detachment [
82,
93].
Ashton et al. introduced the two-phase hypothesis on ROP pathogenesis, demonstrating that exposing healthy cats to 70–80% oxygen for four days induces newly formed capillaries, leading to a process of “vaso-obliteration.” Upon returning to normal air exposure, a phase of “vasoproliferation” is observed [
94]. In the initial phase (phase I), physiological retinal angiogenesis is delayed due to high oxygen exposure, resulting in vascular occlusion, reduced serum IGF-1, and delayed expression of VEGF receptors 2 [
95]. Subsequently (phase II), retinal and vitreous neoangiogenesis occurs alongside increased levels of HIF-1α, VEGF, IGF-1, placental growth factor, erythropoietin (EPO), metalloproteinase (MMP)-2, MMP-9, and angiopoietin (Ang)-2 [
96]. Another model, the oxygen-induced retinopathy (OIR) mouse model, mimics high oxygen levels similar to Ashton’s experiments. In this model, exposure to constant high oxygen (75% O2) causes newly formed capillaries to regress, leading to central areas of vaso-obliteration. Upon returning to room air, relative hypoxia triggers the release of angiogenic factors, promoting the vasoproliferation of blood vessels into the vitreous [
97].
Figure 1 illustrates Phases 1 and 2 in the pathogenesis of ROP, focusing on the distinct expression of key mediators contributing to the onset of the disease.
Figure 1. Schematic representation depicting the two pathogenetic phases in ROP, highlighting distinct expression levels of key mediators in the retina. ROP: retinopathy of prematurity; ROS: reactive oxygen species; O2: oxygen; VEGF: vascular endothelial growth factor; IGF-1: insulin-like growth factor 1; EPO: erythropoietin; HIF-1α: hypoxia-inducible factor 1 alpha; MMP: metalloproteinase; Ang-2: angiopoietin 2. Upward black arrows indicate upregulation or increased concentration, downward black arrows indicate downregulation or decreased concentration.
3. Exploring Molecular Cascades in Retinopathy of Prematurity
Throughout the various pathological stages of ROP, a multitude of molecular signaling pathways emerge, contributing to inflammatory processes and an abundance of ROS and RNS—two crucial factors in the early stages of ROP [
83,
98].
Subsequent sections delve into the primary pathways responsible for vaso-obliteration and vaso-proliferation during ROP pathogenesis.
3.1. The Central Role of Nitro-Oxidative Stress and Inflammatory Factors
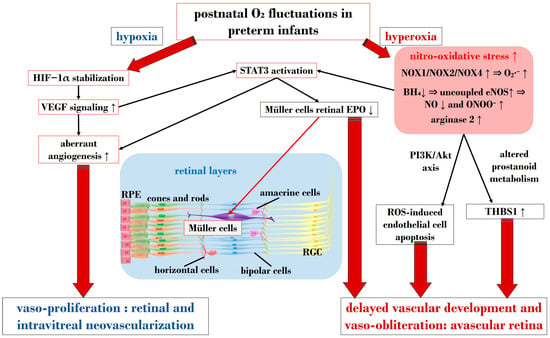
In ROP, endothelial cell apoptosis triggered by oxidative stress is implicated in the process of vaso-obliteration that occurs in the retina during the initial phase of the disorder. Gu et al. demonstrated in bovine retinal endothelial cells that hyperoxia-induced nitro-oxidative stress leads to retinal capillary endothelial cell apoptosis, potentially by impeding growth factor-induced activation of the PI3K/Akt signaling pathway [
99]. The pro-oxidative enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), present in isoforms NOX1, NOX2, and NOX4, generates high levels of superoxide (O
2∙
−), one of the most detrimental ROS, and has been associated with oxidative stress and vascular neoangiogenesis in OIR models [
100,
101,
102]. Wang et al. reported in a rodent model of OIR that NOX4 might regulate intravitreal neovascularization mediated by VEGFR2 via activated signal transducer and activator of transcription (STAT) 3 within endothelial cells [
101]. Studies by Saito et al. on animal models of ROP showcased that hyperoxia activates NOX, leading to an excess of ROS, culminating in apoptosis and neoangiogenesis independently of VEGF [
103,
104]. Furthermore, Byfield et al. demonstrated in a rodent model subjected to repeated oxygen fluctuations that hyperoxia-induced NOX activation leads to intravitreal VEGF-associated vascularization through a Janus tyrosine kinase (JAK)2/STAT3 pathway, and inhibiting this signaling pathway reduced neoangiogenesis [
105]. However, VEGF signaling pathways seem to play a role in both phases of ROP, influencing pro-inflammatory and pro-oxidative processes. The same research group later revealed that VEGF-induced STAT3 activation blocked retinal angiogenesis by downregulating local expression of erythropoietin (EPO) in Müller cells during phase 1 of OIR [
106]. Ren et al.’s investigation in a rodent model exposed to hyperoxia demonstrated that hyperoxia-induced STAT3 signaling enhances hepcidin expression—a key protein involved in iron balance—suggesting a potential compensatory mechanism to counteract iron overload linked to neoangiogenesis and proposing targeting molecules to regulate iron regulation [
107].
Nitric oxide (NO) is pivotal in regulating vasodilation processes. Three main isoforms of NO synthases (NOS)—neural (n)NOS, inducible (i)NOS, and endothelial (e)NOS—are described in the literature. To counteract vaso-obliteration, vasodilation via NO synthesis occurs during the early phases of ROP [
108]. However, differential NO responses are associated with the redox state [
109]. As the disease progresses, excess NO synthesis becomes detrimental, favoring neoangiogenesis [
110]. Importantly, normal NOS activity relies on the availability of the cofactor (6R)-5,6,7,8-tetrahydrobiopterin (BH
4). Edgar et al. assessed in a murine model of OIR that exposure to oxygen led to decreased BH
4 levels in the retinas, lungs, and aortas of mice, resulting in increased NOS-related ROS [
111]. In circumstances of reduced BH
4, uncoupled eNOS activity leads to nitro-oxidative stress in ROP pathogenesis. Specifically, in hyperoxia, impaired eNOS generates peroxynitrite (ONOO
−) rather than physiological NO. Alongside eNOS, iNOS has also been reported as a pathogenetic factor in ROP. For instance, hypoxia-induced activation of iNOS in a murine model of OIR was linked to HIF-1α activation, VEGF expression, and PI3K/Akt signaling during neoangiogenesis, and inhibiting iNOS reduced the expression of these mediators [
112]. However, NO also plays a significant role in neoangiogenesis processes during the vaso-proliferative phase, being fundamental in events of vascular permeability and leakage observed in retinopathies, affecting adherent junctions and endothelial cell polarity [
113,
114].
Notably, nitro-oxidative stress can interfere with prostanoid metabolism, exacerbating vaso-obliteration and contributing to avascular retinal onset. Reactive nitrogen species were observed in a murine OIR model to promote an isomerization of arachidonic acid to trans-arachidonic acid, involved in upregulating the anti-angiogenic factor thrombospondin-1 [
115]. In this context, the role of phospholipase A2 (PLA-2) becomes noteworthy—an enzyme triggered by hypoxia and ROS abundance, influencing prostanoid metabolism via arachidonic acid release [
116]. This molecule acts as a substrate for cyclooxygenase (COX), which converts it into proangiogenic eicosanoids, such as prostaglandins, prostacyclin, and thromboxane. Barnett et al. showed that suppressing PLA-2 decreased proangiogenic prostaglandins and intravitreal neoangiogenesis in an OIR model [
116].
Arginase, present in isoforms arginase 1 and 2, hydrolyzes L-arginine to ornithine and urea, also producing glutamate [
117]. This enzyme has been implicated in neural regeneration and protection [
118]. Preterm infants exhibit low arginine levels, a crucial amino acid for retinal vascular development [
119,
120]. Arginine administration, particularly intravitreally with glutamine, countered neoangiogenesis in an OIR mouse model [
121]. Importantly, arginase functionality and expression are heightened in contexts of inflammation and ROS excess, potentially interfering with NOS activity by competing for the substrate L-arginine. This competition indirectly triggers NOS uncoupling, leading to an overproduction of ONOO
− [
122,
123]. Arginase 1 has been associated with neuroprotection [
124], while arginase 2 might play a role in the events leading to retinal injuries, closely linked with nitro-oxidative stress and inflammation [
125]. Shosha et al. proposed that NOX2-related O
2∙
− induces an upregulation of arginase 2 in ischemia/reperfusion injury, contributing to neurovascular degeneration [
126].
In addition to oxidative stress, inflammatory events play a crucial role in the pathogenesis of ROP [
127]. Cytokines such as IL-1β, tumor necrosis factor-α (TNF-α), and IL-6 are identified as primary drivers of inflammation, capable of inducing the overexpression of various inflammatory mediators, including chemokines and adhesion molecules. Specifically, within the hypoxic neonatal retina, retinal microglia can produce substantial amounts of IL-1β and TNF-α, ultimately promoting the death of retinal ganglion cells [
128]. Furthermore, a study by Rivera and colleagues demonstrated in an OIR model that IL-1β is associated with retinal microvascular degeneration, triggering the release of the proapoptotic/repulsive factor semaphorin-3A from neurons [
129]. Subsequent investigations by the same research group on the same model revealed the early pivotal role of IL-1β in the choroid, contributing to the involution of choroidal blood vessels and causing a loss of retinal pigment epithelium and photoreceptors [
130]. As a consequence of cytokine downstream activation, chemokines also play a role in the pathogenesis of ROP, facilitating chemotaxis and the recruitment of immune cells to sites of inflammation. Specifically, chemokines implicated in ROP include IL-8, “RANTES” (Regulated and Normal T-cell Expressed and Secreted), and monocyte chemotactic protein 1 [
131,
132,
133,
134,
135,
136]. Taken together, inflammatory factors are pivotal in the pathophysiology of ROP, considering their role in orchestrating, together with oxidative stress, an amplification of the aberrant immune-mediated activation that leads to retinal cell death and choroidal degeneration.
3.2. The Crucial Involvement of HIF-1α and VEGF
HIF is a transcription factor composed of two subunits: HIF-1α (or its analogs HIF-2α and HIF-3α) and HIF-1β [
137]. In normoxic conditions, HIF-1α undergoes hydroxylation by a prolyl hydroxylase domain (PHD) in the cytosol [
138]. However, under hypoxic conditions, the enzymatic activities of PHD are inhibited, resulting in an increase in HIF-1α expression. Subsequently, HIF-1α binds to HIF-1β in the nucleus, forming the HIF-1 complex, which activates angiogenic mechanisms to help cells adapt to hypoxia. HIF-1α orchestrates the expression of several neoangiogenic mediators, including VEGF, EPO, angiopoietin (Ang)-1, and Ang-2, all observed to be upregulated in phase 2 of ROP [
139]. Notably, under hypoxic conditions, HIF-1α is pivotal in reprogramming cellular metabolism, enhancing glycolysis, and increasing mitochondrial NADPH synthesis [
140]. Even under normoxic conditions, HIF-1α activity can be induced by ROS and stabilized by inflammatory cytokines and growth factors like IGF-1 and TGF-β [
141,
142]. Enhanced HIF-1α activity due to relative intrauterine hypoxia is critical for physiological retinal vascular development [
143]. However, in premature births, postnatal hyperoxia suppresses HIF-1α activity, leading to reduced VEGF release and consequent retinal capillary obliteration [
144,
145]. Stabilizing HIF-1α might represent a potential molecular target to halt the progression to phase 2 of ROP, as discussed in
Section 4.2.4.
As mentioned earlier, VEGF plays a crucial role in both phases of ROP. Reduced VEGF levels under hyperoxia contribute to vaso-obliteration via endothelial cell apoptosis [
146,
147]. Conversely, in phase 2 under hypoxia, increased retinal VEGF levels act on endothelial cells through a paracrine route [
148]. VEGF’s signaling involves the downregulation of retinal EPO in Müller cells via STAT3 activation. Furthermore, retinal endothelium expresses VEGFR-2, a receptor pivotal in neoangiogenic events and responsible for directing dividing endothelial cells in the developing retina [
149]. Upregulation of VEGFR-2 disrupts dividing endothelial cells, potentially driving them to develop outside the retina, as observed in models of intravitreal neovascularization [
150]. Inhibiting VEGFR-2 has shown promise in reducing intravitreal neoangiogenesis in preclinical investigations using the OIR model [
151].
Figure 2 provides a schematic overview illustrating the primary molecular pathways that unfold during hyperoxia and hypoxia in ROP.
Figure 2. Schematic overview depicting the primary molecular pathways activated during phases 1 and 2 in ROP. ROS: reactive oxygen species; NOX: nicotinamide adenine dinucleotide phosphate oxidase; O2: oxygen; O2∙−: superoxide; ONOO−: peroxynitrite; VEGF: vascular endothelial growth factor; EPO: erythropoietin; HIF-1α: hypoxia-inducible factor 1 alpha; BH4: tetrahydrobiopterin; NO: nitric oxide; eNOS: endothelial nitric oxide synthase; STAT3: signal transducer and activator of transcription 3; RGC: retinal ganglion cell; RPE: retinal pigment epithelium; THBS1: thrombospondin 1. Upward black arrows indicate upregulation or increased concentration, downward black arrows indicate downregulation or decreased concentration.