Liver disease-related mortality is a major cause of death worldwide. Hepatic innate and adaptive immune cells play diverse roles in liver homeostasis and disease. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells. MDSCs can be broadly divided into monocytic MDSCs and polymorphonuclear or granulocytic MDSCs, and they functionally interact with both liver parenchymal and nonparenchymal cells, such as hepatocytes and regulatory T cells, to impact liver disease progression. The infiltration and activation of MDSCs in liver disease can be regulated by inflammatory chemokines and cytokines, tumor-associated fibroblasts, epigenetic regulation factors, and gut microbiota during liver injury and cancer.
- myeloid-derived suppressor cells
- liver inflammation
- fibrosis
- hepatocellular carcinoma
- cell–cell interaction
1. Introduction
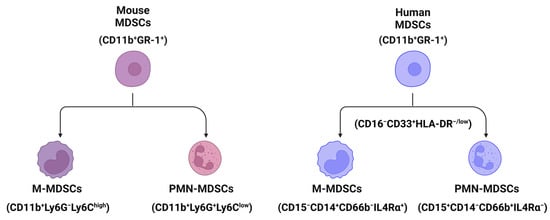
2. The Classification and Markers of MDSCs in Mouse and Human Livers
3. Pathogenesis of MDSCs in Liver Disease
3.1. MDSCs in Liver Inflammation
3.2. MDSCs in Hepatic Cell Death
3.3. MDSCs in Liver Fibrosis and Cirrhosis
3.4. MDSCs in Hepatocarcinogenesis
4. The Interactions of MDSCs with Liver Parenchymal and Nonparenchymal Cells
4.1. Interaction with Parenchymal Cells
4.2. Interaction with Nonparenchymal Cells
5. Factors That Impact MDSC Infiltration and Function during Liver Injury
5.1. Inflammation
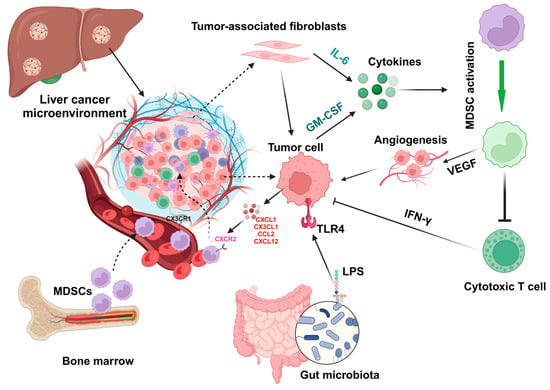
5.2. Chemokines and Cytokines
5.3. Tumor-Associated Fibroblasts
5.4. Epigenetic Regulation
5.5. Gut Microbiota
6. Roles of MDSCs in Different Liver Diseases
6.1. Hepatocellular Carcinoma
6.2. Cholangiocarcinoma
6.3. Metastatic Liver Cancer
6.4. Subcutaneous Liver Cancer
6.5. Liver Regenration
6.6. Autoimmune Hepatitis
6.7. Alcoholic and Nonalcoholic Liver Diseases
7. Summary
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines12020299
References
- Golabi, P.; Paik, J.M.; Eberly, K.; de Avila, L.; Alqahtani, S.A.; Younossi, Z.M. Causes of death in patients with Non-alcoholic Fatty Liver Disease (NAFLD), alcoholic liver disease and chronic viral Hepatitis B and C. Ann. Hepatol. 2022, 27, 100556.
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986.
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171.
- Tapper, E.B.; Parikh, N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: Observational study. BMJ 2018, 362, k2817.
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376.
- Konyn, P.; Ahmed, A.; Kim, D. Current epidemiology in hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1295–1307.
- Zhang, C.; Yang, M. The Emerging Factors and Treatment Options for NAFLD-Related Hepatocellular Carcinoma. Cancers 2021, 13, 3740.
- Heymann, F.; Tacke, F. Immunology in the liver—From homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110.
- Byun, J.S.; Yi, H.S. Hepatic Immune Microenvironment in Alcoholic and Nonalcoholic Liver Disease. BioMed Res. Int. 2017, 2017, 6862439.
- Kefalakes, H.; Horgan, X.J.; Jung, M.K.; Amanakis, G.; Kapuria, D.; Bolte, F.J.; Kleiner, D.E.; Koh, C.; Heller, T.; Rehermann, B. Liver-Resident Bystander CD8(+) T Cells Contribute to Liver Disease Pathogenesis in Chronic Hepatitis D Virus Infection. Gastroenterology 2021, 161, 1567–1583.e1569.
- Zhou, J.Y. Innate immunity and early liver inflammation. Front. Immunol. 2023, 14, 1175147.
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276.
- Zhang, C.Y.; Liu, S.; Yang, M. Regulatory T cells and their associated factors in hepatocellular carcinoma development and therapy. World J. Gastroenterol. 2022, 28, 3346–3358.
- Su, Q.; Kim, S.Y.; Adewale, F.; Zhou, Y.; Aldler, C.; Ni, M.; Wei, Y.; Burczynski, M.E.; Atwal, G.S.; Sleeman, M.W.; et al. Single-cell RNA transcriptome landscape of hepatocytes and non-parenchymal cells in healthy and NAFLD mouse liver. iScience 2021, 24, 103233.
- Ramachandran, P.; Dobie, R.; Wilson-Kanamori, J.R.; Dora, E.F.; Henderson, B.E.P.; Luu, N.T.; Portman, J.R.; Matchett, K.P.; Brice, M.; Marwick, J.A.; et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 2019, 575, 512–518.
- Wang, J.; Hu, W.; Shen, Z.; Liu, T.; Dai, W.; Shen, B.; Li, X.; Wu, J.; Lu, L.; Li, S.; et al. Dissecting the single-cell transcriptomeunderlying chronic liver injury. Mol. Ther. Nucleic Acids 2021, 26, 1364–1373.
- Kalathil, S.G.; Thanavala, Y. Importance of myeloid derived suppressor cells in cancer from a biomarker perspective. Cell Immunol. 2021, 361, 104280.
- Youn, J.I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008, 181, 5791–5802.
- Ostrand-Rosenberg, S.; Fenselau, C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J. Immunol. 2018, 200, 422–431.
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64.
- Yang, Y.M.; Kim, S.Y.; Seki, E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin. Liver Dis. 2019, 39, 26–42.
- D’Souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783.
- Montanari, N.R.; Ramírez, R.; Aggarwal, A.; van Buuren, N.; Doukas, M.; Moon, C.; Turner, S.; Diehl, L.; Li, L.; Debes, J.D.; et al. Multi-parametric analysis of human livers reveals variation in intrahepatic inflammation across phases of chronic hepatitis B infection. J. Hepatol. 2022, 77, 332–343.
- Ganz, M.; Bukong, T.N.; Csak, T.; Saha, B.; Park, J.K.; Ambade, A.; Kodys, K.; Szabo, G. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J. Transl. Med. 2015, 13, 193.
- Govaere, O.; Petersen, S.K.; Martinez-Lopez, N.; Wouters, J.; Van Haele, M.; Mancina, R.M.; Jamialahmadi, O.; Bilkei-Gorzo, O.; Lassen, P.B.; Darlay, R.; et al. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 1001–1012.
- Ren, R.; He, Y.; Ding, D.; Cui, A.; Bao, H.; Ma, J.; Hou, X.; Li, Y.; Feng, D.; Li, X.; et al. Aging exaggerates acute-on-chronic alcohol-induced liver injury in mice and humans by inhibiting neutrophilic sirtuin 1-C/EBPα-miRNA-223 axis. Hepatology 2022, 75, 646–660.
- Zhu, K.; Zhang, N.; Guo, N.; Yang, J.; Wang, J.; Yang, C.; Yang, C.; Zhu, L.; Xu, C.; Deng, Q.; et al. SSChighCD11bhighLy-6ChighLy-6Glow myeloid cells curtail CD4 T cell response by inducible nitric oxide synthase in murine hepatitis. Int. J. Biochem. Cell Biol. 2014, 54, 89–97.
- Song, P.; Zhang, J.; Zhang, Y.; Shu, Z.; Xu, P.; He, L.; Yang, C.; Zhang, J.; Wang, H.; Li, Y.; et al. Hepatic recruitment of CD11b+Ly6C+ inflammatory monocytes promotes hepatic ischemia/reperfusion injury. Int. J. Mol. Med. 2018, 41, 935–945.
- Luedde, T.; Kaplowitz, N.; Schwabe, R.F. Cell Death and Cell Death Responses in Liver Disease: Mechanisms and Clinical Relevance. Gastroenterology 2014, 147, 765–783.e764.
- Zhang, C.; Sui, Y.; Liu, S.; Yang, M. Molecular mechanisms of metabolic disease-associated hepatic inflammation in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Explor. Dig. Dis. 2023, 2, 246–275.
- Hsu, C.Y.; Lin, Y.C.; Chang, L.Y.; Huang, S.K.; Huang, C.H.; Yang, C.K.; Huang, C.T.; Lin, C.Y. Therapeutic Role of Inducible Nitric Oxide Synthase Expressing Myeloid-Derived Suppressor Cells in Acetaminophen-Induced Murine Liver Failure. Front. Immunol. 2020, 11, 574839.
- Suh, Y.G.; Kim, J.K.; Byun, J.S.; Yi, H.S.; Lee, Y.S.; Eun, H.S.; Kim, S.Y.; Han, K.H.; Lee, K.S.; Duester, G.; et al. CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice. Hepatology 2012, 56, 1902–1912.
- Höchst, B.; Mikulec, J.; Baccega, T.; Metzger, C.; Welz, M.; Peusquens, J.; Tacke, F.; Knolle, P.; Kurts, C.; Diehl, L.; et al. Differential induction of Ly6G and Ly6C positive myeloid derived suppressor cells in chronic kidney and liver inflammation and fibrosis. PLoS ONE 2015, 10, e0119662.
- Gao, M.; Huang, A.; Sun, Z.; Sun, Y.; Chang, B.; Zhang, J.Y.; Zou, Z.S. Granulocytic myeloid-derived suppressor cell population increases with the severity of alcoholic liver disease. J. Cell. Mol. Med. 2019, 23, 2032–2041.
- Liu, H.; Yeung, W.H.O.; Pang, L.; Liu, J.; Liu, X.B.; Pan Ng, K.T.; Zhang, Q.; Qiu, W.Q.; Zhu, Y.; Ding, T.; et al. Arachidonic acid activates NLRP3 inflammasome in MDSCs via FATP2 to promote post-transplant tumour recurrence in steatotic liver grafts. JHEP Rep. 2023, 5, 100895.
- Liu, H.; Ling, C.C.; Yeung, W.H.O.; Pang, L.; Liu, J.; Zhou, J.; Zhang, W.Y.; Liu, X.B.; Ng, T.P.K.; Yang, X.X.; et al. Monocytic MDSC mobilization promotes tumor recurrence after liver transplantation via CXCL10/TLR4/MMP14 signaling. Cell Death Dis. 2021, 12, 489.
- Medina-Echeverz, J.; Eggert, T.; Han, M.; Greten, T.F. Hepatic myeloid-derived suppressor cells in cancer. Cancer Immunol. Immunother. 2015, 64, 931–940.
- Liepelt, A.; Tacke, F. Stromal cell-derived factor-1 (SDF-1) as a target in liver diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G203–G209.
- Eggert, T.; Wolter, K.; Ji, J.; Ma, C.; Yevsa, T.; Klotz, S.; Medina-Echeverz, J.; Longerich, T.; Forgues, M.; Reisinger, F.; et al. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell 2016, 30, 533–547.
- Liu, G.; Wang, Q.; Deng, L.; Huang, X.; Yang, G.; Cheng, Q.; Guo, T.; Guo, L.; Niu, C.; Yang, X.; et al. Hepatic RACK1 deficiency protects against fulminant hepatitis through myeloid-derived suppressor cells. Theranostics 2022, 12, 2248–2265.
- Kapanadze, T.; Gamrekelashvili, J.; Ma, C.; Chan, C.; Zhao, F.; Hewitt, S.; Zender, L.; Kapoor, V.; Felsher, D.W.; Manns, M.P.; et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J. Hepatol. 2013, 59, 1007–1013.
- Tian, S.; Liao, L.; Zhou, Q.; Huang, X.; Zheng, P.; Guo, Y.; Deng, T.; Tian, X. Curcumin inhibits the growth of liver cancer by impairing myeloid-derived suppressor cells in murine tumor tissues. Oncol. Lett. 2021, 21, 286.
- Xu, Y.; Fang, F.; Jiao, H.; Zheng, X.; Huang, L.; Yi, X.; Zhao, W. Activated hepatic stellate cells regulate MDSC migration through the SDF-1/CXCR4 axis in an orthotopic mouse model of hepatocellular carcinoma. Cancer Immunol. Immunother. 2019, 68, 1959–1969.
- He, Q.; Liu, M.; Huang, W.; Chen, X.; Zhang, B.; Zhang, T.; Wang, Y.; Liu, D.; Xie, M.; Ji, X.; et al. IL-1β-Induced Elevation of Solute Carrier Family 7 Member 11 Promotes Hepatocellular Carcinoma Metastasis Through Up-regulating Programmed Death Ligand 1 and Colony-Stimulating Factor 1. Hepatology 2021, 74, 3174–3193.
- Yu, S.J.; Ma, C.; Heinrich, B.; Brown, Z.J.; Sandhu, M.; Zhang, Q.; Fu, Q.; Agdashian, D.; Rosato, U.; Korangy, F.; et al. Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma. J. Hepatol. 2019, 70, 449–457.
- Yang, H.; Kang, B.; Ha, Y.; Lee, S.H.; Kim, I.; Kim, H.; Lee, W.S.; Kim, G.; Jung, S.; Rha, S.Y.; et al. High serum IL-6 correlates with reduced clinical benefit of atezolizumab and bevacizumab in unresectable hepatocellular carcinoma. JHEP Rep. 2023, 5, 100672.
- Lin, Y.; Yang, X.; Liu, W.; Li, B.; Yin, W.; Shi, Y.; He, R. Chemerin has a protective role in hepatocellular carcinoma by inhibiting the expression of IL-6 and GM-CSF and MDSC accumulation. Oncogene 2017, 36, 3599–3608.
- Liu, M.; Zhou, J.; Liu, X.; Feng, Y.; Yang, W.; Wu, F.; Cheung, O.K.-W.; Sun, H.; Zeng, X.; Tang, W. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut 2020, 69, 365–379.
- Mann, D.A. Epigenetics in liver disease. Hepatology 2014, 60, 1418–1425.
- Habash, N.W.; Sehrawat, T.S.; Shah, V.H.; Cao, S. Epigenetics of alcohol-related liver diseases. JHEP Rep. 2022, 4, 100466.
- Zhu, Z.Y.; Tang, N.; Wang, M.F.; Zhou, J.C.; Wang, J.L.; Ren, H.Z.; Shi, X.L. Comprehensive Pan-Cancer Genomic Analysis Reveals PHF19 as a Carcinogenic Indicator Related to Immune Infiltration and Prognosis of Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 781087.
- Wang, L.; Zhu, R.; Huang, Z.; Li, H.; Zhu, H. Lipopolysaccharide-induced toll-like receptor 4 signaling in cancer cells promotes cell survival and proliferation in hepatocellular carcinoma. Dig. Dis. Sci. 2013, 58, 2223–2236.
- Gupta, R.; Kadhim, M.M.; Turki Jalil, A.; Obayes, A.M.; Aminov, Z.; Alsaikhan, F.; Ramírez-Coronel, A.A.; Ramaiah, P.; Tayyib, N.A.; Luo, X. Multifaceted role of NF-κB in hepatocellular carcinoma therapy: Molecular landscape, therapeutic compounds and nanomaterial approaches. Environ. Res. 2023, 228, 115767.
- Cao, M.; Xu, Y.; Youn, J.-i.; Cabrera, R.; Zhang, X.; Gabrilovich, D.; Nelson, D.R.; Liu, C. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab. Investig. 2011, 91, 598–608.
- Xiong, Z.; Chan, S.L.; Zhou, J.; Vong, J.S.L.; Kwong, T.T.; Zeng, X.; Wu, H.; Cao, J.; Tu, Y.; Feng, Y.; et al. Targeting PPAR-gamma counteracts tumour adaptation to immune-checkpoint blockade in hepatocellular carcinoma. Gut 2023, 72, 1758–1773.
- Loeuillard, E.; Yang, J.; Buckarma, E.; Wang, J.; Liu, Y.; Conboy, C.; Pavelko, K.D.; Li, Y.; O’Brien, D.; Wang, C.; et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J. Clin. Investig. 2020, 130, 5380–5396.
- Lin, Y.; Li, B.; Yang, X.; Cai, Q.; Liu, W.; Tian, M.; Luo, H.; Yin, W.; Song, Y.; Shi, Y.; et al. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia 2019, 21, 1133–1142.
- Lin, Q.; Ren, L.; Jian, M.; Xu, P.; Li, J.; Zheng, P.; Feng, Q.; Yang, L.; Ji, M.; Wei, Y.; et al. The mechanism of the premetastatic niche facilitating colorectal cancer liver metastasis generated from myeloid-derived suppressor cells induced by the S1PR1–STAT3 signaling pathway. Cell Death Dis. 2019, 10, 693.
- Ghosh, C.C.; Heatherton, K.R.; Connell, K.P.O.; Alexander, I.S.; Greer, D.A.; LaPorte, J.; Guha, P.; Cox, B.F.; Katz, S.C. Regional infusion of a class C TLR9 agonist enhances liver tumor microenvironment reprogramming and MDSC reduction to improve responsiveness to systemic checkpoint inhibition. Cancer Gene Ther. 2022, 29, 1854–1865.
- Zhang, M.; Wang, L.; Liu, W.; Wang, T.; De Sanctis, F.; Zhu, L.; Zhang, G.; Cheng, J.; Cao, Q.; Zhou, J.; et al. Targeting Inhibition of Accumulation and Function of Myeloid-Derived Suppressor Cells by Artemisinin via PI3K/AKT, mTOR, and MAPK Pathways Enhances Anti-PD-L1 Immunotherapy in Melanoma and Liver Tumors. J. Immunol. Res. 2022, 2022, 2253436.
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55.
- Nachmany, I.; Bogoch, Y.; Sivan, A.; Amar, O.; Bondar, E.; Zohar, N.; Yakubovsky, O.; Fainaru, O.; Klausner, J.M.; Pencovich, N. CD11b+ Ly6G+ myeloid-derived suppressor cells promote liver regeneration in a murine model of major hepatectomy. FASEB J. 2019, 33, 5967–5978.
- Li, B.; Lian, M.; Li, Y.; Qian, Q.; Zhang, J.; Liu, Q.; Tang, R.; Ma, X. Myeloid-derived suppressive cells deficient in liver X receptor α protected from autoimmune hepatitis. Front. Immunol. 2021, 12, 732102.
- Zhang, C.; Liu, S.; Yang, M. Hepatocellular Carcinoma and Obesity, Type 2 Diabetes Mellitus, Cardiovascular Disease: Causing Factors, Molecular Links, and Treatment Options. Front. Endocrinol. 2021, 12, 808526.
- French, S.W. Epigenetic events in liver cancer resulting from alcoholic liver disease. Alcohol. Res. 2013, 35, 57–67.
- Li, S.; Wang, N.; Tan, H.Y.; Hong, M.; Yuen, M.F.; Li, H.; Feng, Y. Expansion of Granulocytic, Myeloid-Derived Suppressor Cells in Response to Ethanol-Induced Acute Liver Damage. Front. Immunol. 2018, 9, 1524.
