The effects of different types of biostimulants on crops include improving the visual quality of the final products, stimulating the immune systems of plants, inducing the biosynthesis of plant defensive biomolecules, removing heavy metals from contaminated soil, improving crop performance, reducing leaching, improving root development and seed germination, inducing tolerance to abiotic and biotic stressors, promoting crop establishment and increasing nutrient-use efficiency. Protein hydrolysates are mixtures of polypeptides and free amino acids resulting from enzymatic and chemical hydrolysis of agro-industrial protein by-products obtained from animal or plant origins, and they are able to alleviate environmental stress effects, improve growth, and promote crop productivity. Amino acids involve various advantages such as increased yield and yield components, increased nutrient assimilation and stress tolerance, and improved yield components and quality characteristics.
- amino acids
- biostimulants
- medicinal plants
- phenols
- protein hydrolysates
1. Introduction
2. Amino Acids
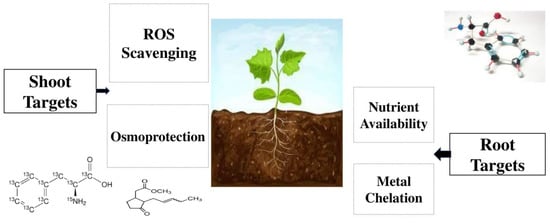
3. Protein Hydrolysates
4. Phenols and Phenolic Biostimulants
This entry is adapted from the peer-reviewed paper 10.3390/plants13020210
References
- La Bella, S.; Consentino, B.B.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Sabatino, L. Impact of Ecklonia maxima seaweed extract and Mo foliar treatments on biofrotification, spinach yield, quality and NUE. Plants 2021, 10, 1139.
- Mashamaite, C.V.; Ngcobo, B.L.; Manyevere, A.; Bertling, I.; Fawole, O.A. Assessing the usefulness of Moringa oleifera leaf extract as a biostimulant to supplement synthetic fertilizers: A review. Plants 2022, 11, 2214.
- Sun, W.; Shahrajabian, M.H.; Lin, M. Research progress of fermented functional foods and protein factory-microbial fermentation technology. Fermentation 2022, 8, 688.
- Sun, W.; Shahrajabian, M.H. The application of arbuscular mycorrhizal fungi as microbial biostimulant, sustainable approaches in modern agriculture. Plants 2023, 12, 3101.
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants-natural health products for human health. Molecules 2023, 28, 1845.
- Sun, W.; Shahrajabian, M.H.; Petropoulos, S.A.; Shahrajabian, N. Developing sustainable agriculture systems in medicinal and aromatic plant production by using chitosan and chitin-based biostimulants. Plants 2023, 12, 2469.
- Rodriguez-Morgado, B.; Gomez, I.; Parrado, J.; Garcia-Martinez, A.M.; Aragon, C.; Tejada, M. Obtaining edaphic biostimulants/biofertilizers from different sewage sludges. Effects on soil biological properties. Environ. Technol. 2015, 36, 2217–2226.
- Orts, A.; Tejada, M.; Parrado, J.; Paneque, P.; Garcia, C.; Hernandez, T.; Gomez-Parrales, I. Production of biostimulants from okara through enzymatic hydrolysis and fermentation with Bacillus licheniformis: Comparative effect on soil biological properties. Environ. Technol. 2019, 40, 2073–2084.
- Abdelkader, M.M.; Gaplaev, M.S.; Terekbaev, A.A.; Puchkov, M.Y. The influence of biostimulants on tomato plants cultivated under hydroponic systems. J. Hortic. Res. 2021, 29, 107–116.
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S.
- Alcazar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249.
- Shahrajabian, M.H.; Sun, W. Various techniques for molecular and rapid detection of infectious and epidemic diseases. Lett. Org. Chem. 2023, 20, 779–801.
- Shahrajabian, M.H.; Sun, W. Survey on multi-omics, and multi-omics data analysis, integration and application. Curr. Pharm. Anal. 2023, 19, 267–281.
- Shahrajabian, M.H.; Petropoulos, S.A.; Sun, W. Survey of the influences of microbial biostimulants on horticultural crops: Case studies and successful paradigms. Horticulturae 2023, 9, 193.
- Aghaye Noroozlo, Y.; Souri, M.K.; Delshad, M. Stimulation effects of foliar application of glycine and glutamine amino acids on growth and quality of sweet basil. Adv. Hortic. Sci. 2019, 33, 495–501.
- Ayyat, A.M.; Kenawy, A.G.M.; Aboel-Ainin, M.A.; Abdel-Mola, M.A.M. Improving growth, productivity and oil yield of Nigella sativa L. Plants by foliar spraying with some stimulants. J. Plant Prod. 2021, 12, 339–344.
- Elsayed, A.A.A.; El-Gohary, A.E.; Khalid, K.A.; Ahmed, A.M.A. Changes in bitter fennel essential oils exposed to foliar spray with L-phenylalanine. Egypt. J. Bot. 2022, 62, 241–253.
- Rafiee, H.; Mehrafarin, A.; Qaderi, A.; Jari, S.K.; Badi, H.N. Phytochemical, agronomical and morphological response of pot marigold (Calendula officinalis L.) to foliar application of biostimulators (Bioactive amino acid compounds). J. Med. Plants 2013, 12, 48–61.
- Mehrabi, S.; Mehrafarin, A.; Badi, H.N. Clarifying the role of methanol and amino acids application on savory (Satureja hortensis L. ). Ann. Biol. Res. 2013, 4, 190–195.
- Hendawy, S.F.; Hussein, M.S.; El-Gohary, A.E.; Ibrahim, M.E. Effect of foliar organic fertilization on the growth, yield and oil content of Mentha piperita var. citrata. Asian J. Agric. Res. 2015, 9, 237–248.
- Shafie, F.; Bayat, H.; Aminifard, M.H.; Saeid Daghighi, S. Biostimulant effects of seaweed extract and amino acids on growth, antioxidants, and nutrient content of Yarrow (Achillea millefolium L.) in the field and greenhouse conditions. Commun. Soil Sci. Plant Anal. 2021, 52, 964–975.
- Wafaa, H.A.A.A.; Rania, M.R.K.; El-Shafay, R.M.M. Effect of spraying with extracts of plants and amino acids on growth and productivity on Coriandrum sativum plants under Shalateen condition. Plant Arch. 2021, 21, 300–307.
- Wahba, H.E.; Motawe, H.M.; Ibrahim, A.Y. Growth and chemical of Urtica pilulifera L. plant as influenced by foliar application of some amino acids. J. Mater. Environ. Sci. 2015, 6, 499–506.
- Shahrajabian, M.H.; Sun, W.; Shen, H.; Cheng, Q. Chinese herbal medicine for SARS and SARS-CoV-2 treatment and prevention, encouraging using herbal medicine for COVID-19 outbreak. Acta Agric. Scand. Sec. B. Soil Plant Sci. 2020, 70, 437–443.
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Traditional herbal medicine for the prevention and treatment of cold and flu in the autumn of 2020, overlapped with COVID-19. Nat. Prod. Commun. 2020, 15, 1934578X20951431.
- Islam, M.; Huang, Y.; Islam, S.; Fan, B.; Tong, L.; Wang, F. Influence of the degree of hydrolysis on functional properties and antioxidant activity of enzymatic soybean protein hydrolysates. Molecules 2022, 27, 6110.
- Peslerbes, M.; Fellenberg, A.; Jardin, J.; Deglaire, A.; Ibanez, R.A. Manufacture of whey protein hydrolysates using plant enzymes: Effect of processing conditions and simulated gastrointestinal digestion on angiotensin-I-converting enzyme (ACE) inhibitory activity. Foods 2022, 11, 2429.
- Cosovanu, D.; Acosta, A.M.; Lopez, P.C.; Gernaey, K.V.; Li, Q.; Lametsch, R.; Canela-Garayoa, R.; Eras, J.; Villorbina, G. Rendered-protein hydrolysates as a low-cost nitrogen source for the fungal biotransformation of 5-hydroxymethylfurfural. Catalysts 2022, 12, 839.
- Aluko, R.E. Amino acids, peptides, and proteins as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Elsevier Inc.: Cambridge, UK, 2015; pp. 105–140.
- Makhaye, G.; Aremu, A.O.; Gerrano, A.S.; Tesfay, S.; Du Plooy, C.P.; Amoo, S.O. Biopriming with seaweed extract and microbial-based commercial biostimulants influences seed germination of five Abelmoschus esculentus genotypes. Plants 2021, 10, 1327.
- Martinez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212.
- Ebinezer, L.B.; Franchin, C.; Trentin, A.R.; Carletti, P.; Trevisan, S.; Agrawal, G.K.; Rakwal, R.; Quaggiotti, S.; Arriogoni, G.; Masi, A. Quantitative proteomics of maize roots treated with a protein hydrolysate: A comparative study with transcriptomics highlights the molecular mechanisms responsive to biostimulants. J. Agric. Food Chem. 2020, 68, 7541–7553.
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671.
- Baqer, H.A.A.-R.; Zeboon, N.; Al-Behadili, A. The tole and importance of amino acids within plants: A review. Plant Arch. 2019, 19, 1402–1410.
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41.
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein hydrolysate or plant extract-based biostimulants enhanced yield and quality performances of greenhouse perennial wall rocket grown in different seasons. Plants 2019, 8, 208.
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42.
- Chai, T.-T.; Ee, K.-Y.; Kumar, D.T.; Manan, F.A.; Wong, F.-C. Plant bioactive peptides: Current status and prospects towards use on human health. Protein Pept. Lett. 2021, 28, 623–642.
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57.
- Ryu, B.; Shin, K.-H.; Kim, S.-K. Muscle protein hydrolysates and amino acid composition in fish. Mar. Drugs 2021, 19, 377.
- Siddik, M.A.B.; Howieson, J.; Fotedar, R.; Partridge, G.J. Enzymatic fish protein hydrolysates in finfish aquaculture: A review. Rev. Aquac. 2021, 13, 406–430.
- Sierra Lopera, L.M.; Sepulveda Rincon, C.T.; Vasquez Mazo, P.; Figueroa Moreno, O.A.; Zapata Montoya, J.E. Byproducts of aquaculture processes: Development and prospective uses. Review. Vitae 2018, 25, 128–140.
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Using bacteria and fungi as plant biostimulants for sustainable agricultural production systems. Rec. Pat. Biotechnol. 2022, 17, 206–244.
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Foliar application of nutrients on medicinal and aromatic plants, the sustainable approaches for higher and better production. Beni-Suef Uni. J. Basic Appl. Sci. 2022, 11, 26.
- Shahrajabian, M.H.; Sun, W. Mechanism of action of collagen and epidermal growth factor: A review on theory and research methods. Mini Rev. Med. Chem. 2023, 23, 453–477.
- Shahrajabian, M.H.; Sun, W. Five important seeds in traditional medicine, and pharmacological benefits. Seeds 2023, 2, 290–308.
- Shahrajabian, M.H.; Sun, W. The golden spice for life: Turmeric with the pharmacological benefits of curcuminoids components, including curcumin, bisdemethoxycurcumin, and demethoxycurcumin. Curr. Org. Synth. 2023, 20.
- Shahrajabian, M.H.; Kuang, Y.; Cui, H.; Fu, L.; Sun, W. Metabolic changes of active components of important medicinal plants on the basis of traditional Chinese medicine under different envrionmental stresses. Curr. Org. Chem. 2023, 27, 782–806.
- Sierras, N.; Botta, A.; Staasing, L.; Martinez, M.J.; Bru, R. Understanding the effect of amino acids based biostimulant by an enantiomeric analysis of their active principles and a proteomic profiling approach. Acta Hortic. 2016, 1148, 93–100.
- Atilio, J.B.; Causin, H.F. The central role of amino acids on nitrogen utilization and plant growth. J. Plant Physiol. 1996, 149, 358–362.
- Rai, V.K. Role of amino acids in plant responses to stresses. Biol. Plant. 2002, 45, 481–487.
- Shahrajabian, M.H.; Sun, W.; Soleymani, A.; Cheng, Q. Traditional herbal medicines to overcome stress, anxiety and improve mental health in outbreaks of human coronaviruses. Phytother. Res. 2020, 2020, 1237–1247.
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819.
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants application: A low input cropping management tool for sustainable farming vegetables. Biomolecules 2021, 11, 698.
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Different methods for molecular and rapid detection of human novel coronavirus. Curr. Pharm. Des. 2021, 27, 2893–2903.
- Shahrajabian, M.H.; Sun, W. Sustainable approaches to boost yield and chemical constituents of aromatic and medicinal plants by application of biostimulants. Recent Pat. Food Nutr. Agric. 2022, 13, 72–92.
- Shahrajabian, M.H.; Cheng, Q.; Sun, W. The effects of amino acids, phenols and protein hydrolysates as biostimulants on sustainable crop production and alleviated stress. Rec. Pat. Biotechnol. 2022, 16, 319–328.
- Cheng, Y.; Tian, Q.; Zhang, W.-H. Glutamate receptors are involved in mitigating effects of amino acids on seed germination of Arabidopsis thaliana under salt stress. Environ. Exp. Bot. 2016, 130, 68–78.
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The synthesis and the role of B-alanine in plants. Front. Plant Sci. 2019, 10, 921.
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of basil (Ocimum bacilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970.
- Paleckiene, R.; Sviklas, A.; Slinksiene, R. Physicochemical properties of a microelement fertilizer with amino acids. Russ. J. Appl. Chem. 2007, 80, 352–357.
- Johansson, A. Conversations on chelation and mineral nutrition. Aust. J. Grape Wine Res. 2008, 583, 53–56.
- Seadh, S.R.; El-Abady, M.I.; Farouk, S.; El-Saidy Amal, E.A. Effect of foliar nutrition with humic and amino acids under N-levels on wheat productivity and quality of grains and seeds. Egypt. J. Appl. Sci. 2008, 23, 543–558.
- Toscano, S.; Gomez-Bellot, M.J.; Romano, D.; Sanchez-Blanco, M.J. Physiological and biochemical changes in response to Moringa oleifera biostimulant in petunia plants under water deficit. Sci. Hortic. 2023, 319, 112187.
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Gorecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 2018, 23, 470.
- Amin, A.A.; Gharib, F.A.; El-Awadi, M.; Rashad, E.-S.M. Physiological response of onion plants to foliar application of putrescine and glutamine. Sci. Hortic. 2011, 129, 353–360.
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208.
- Das, C.; Sengupta, T.; Chattopadhyay, S.; Setua, M.; Das, N.K.; Saratchandra, B. Involvement of kinetin and spermidine in controlling salinity stress in mulberry (Morus alba L. cv. S1). Acta Physiol. Plant. 2002, 24, 53–57.
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous application of amino acids improves the growth and yield of lettuce by enhancing photosynthetic assimilation and nutrient availability. Agronomy 2019, 9, 266.
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105.
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53.
- Musbah, H.M.; Ibrahim, K.M. Effects of feeding tyrosine or phenylalanine on the accumulation of polyphenols in Coleus blumei in vivo and in vitro. J. Biotechnol. Res. Cent. 2019, 13, 35–43.
- Reham, M.S.; Khattab, M.E.; Ahmed, S.S.; Kandil, M.A.M. Influence of foliar spray with phenylalanine and nickel on growth, yield quality, and chemical composition of genoveser basil plant. Afr. J. Agric. Res. 2016, 14, 934–941.
- Baharlou, M.J.; Pirbalouti, A.G.; Malekpoor, F. Effect of different concentrations of L-phenylalanine on chemical compositions and yield of essential oil of lemon balm (Melissa officinalis). J. Herb. Drugs 2019, 10, 175–183.
- Noviyanti, R.; Sari, R.L.K.; Kristanti, A.N.; Yachya, A.; Manuhara, Y.S.W. Biomass and flavonoid production of gynura procumbens adventitious roots induced by sucrose, phenylalanine, and tyrosine. Biosci. Res. 2017, 14, 934–941.
- Portu, J.; Santamaria, P.; Lopez, R.; Garde-Cerdan, T. Phenolic composition of Tempranillo grapes following foliar applications of phenylalanine and urea: A two-year study. Sci. Hortic. 2017, 219, 191–199.
- Feng, Z.; Xie, X.; Wu, P.; Chen, M.; Qin, Y.; Zhou, Y.; Zhu, H.; Yao, Q. Phenylalanine-mediated changes in the soil bacterial community promote nitrogen cycling and plant growth. Microbiol. Res. 2023, 275, 127447.
- Ramzan, T.; Shahbaz, M.; Maqsood, M.F.; Zulfiqar, U.; Saman, R.U.; Lili, N.; Irshad, M.; Maqsood, S.; Haider, A.; Shahzad, B.; et al. Phenylalanine supply alleviates the drought stress in mustard (Brassica campestris) by modulating plant growth, photosynthesis, and antioxidant defense system. Plant Physiol. Biochem. 2023, 201, 107828.
- Roman, S.M.-S.; Garde-Cerdan, T.; Baroja, E.; Rubio-Breton, P.; Perez-Alvarez, E.P. Foliar application of phenylalanine plus methyl jasmonate as a tool to improve Grenache grape aromatic composition. Sci. Hortic. 2020, 272, 109515.
- Portu, J.; Lopez-Alfaro, I.; Gomez-Alonso, S.; Lopez, R.; Garde-Cerdan, T. Changes on grape phenolic composition induced by grapevine foliar applications of phenylalanine and urea. Food Chem. 2015, 180, 171–180.
- Lin, H.-C.; Alashi, A.M.; Aluko, R.E.; Pan, B.S.; Chang, Y.-W. Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates. Food Nutr. Res. 2017, 61, 1391666.
- Czelej, M.; Czernecki, T.; Garbacz, K.; Wawrzykowski, J.; Jamiol, M.; Michalak, K.; Walczak, N.; Wilk, A.; Wasko, A. Egg yolk as a new source of peptides with antioxidant and antimicrobial properties. Foods 2023, 12, 3394.
- Bello, A.S.; Ben-Hamadou, R.; Hamdi, H.; Saadaoui, I.; Ahmed, T. Application of cyanobacteria (Roholtiella sp.) liquid extract for the alleviation of salt stress in bell pepper (Capsiucum annuum L.) plants grown in a soilless system. Plants 2022, 11, 104.
- Chan, Y.-J.; Lu, W.-C.; Lin, H.-Y.; Wu, Z.-R.; Liou, C.-W.; Li, P.-H. Effect of rice protein hydrolysates as an egg replacement on the physicochemical properties of flaky egg rolls. Foods 2020, 9, 245.
- Liao, X.; Zhu, Z.; Wu, S.; Chen, M.; Huang, R.; Wang, J.; Wu, Q.; Ding, Y. Preparation of antioxidant protein hydrolysates from Pleurotus geesterans and their protective effects on H2O2 oxidative damaged PC12 cells. Molecules 2020, 25, 5408.
- Leni, G.; Soetemans, L.; Caligiani, A.; Sforza, S.; Bastiaens, L. Degree of hydrolysis affects the techno-functional properties of lesser mealworm protein hydrolysates. Foods 2020, 9, 381.
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81.
- Kanbargi, K.D.; Sonawane, S.K.; Arya, S.S. Encapsulation characteristics of protein hydrolyaste extracted from Ziziphus jujube seed. Int. J Food Prop. 2017, 20, 3215–3224.
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Organic waste utilization and urban food waste composting strategies in China—A review. Not. Sci. Biol. 2021, 13, 10881.
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Archaea, bacteria and termite, nitrogen fixation and sustainable plants production. Not. Bot. Horti Agrobot. 2021, 49, 1–32.
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Barberry (Berberis vulgaris), a medicinal fruit and food with traditional and modern pharmaceutical uses. Isr. J. Plant Sci. 2021, 68, 61–71.
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Fenugreek cultivation with emphasis on historical aspects and its uses in traditional medicine and modern pharmaceutical science. Mini Rev. Med. Chem. 2021, 21, 724–730.
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Natural dietary and medicinal plants with anti-obesity therapeutics activities for treatment and prevention of obesity during lock down and in post-COVID-19 era. Appl. Sci. 2021, 11, 7889.
- Sun, W.; Shahrajabian, M.H. The effectiveness of Rhizobium bacteria on soil fertility and sustainable crop production under cover and catch crops management and green manuring. Not. Bot. Horti Agrobot. 2022, 50, 12560.
- Tchorbanov, B.; Iliev, I.; Litchev, V. Enzymic protein hydrolysates. Biotechnol. Biotechnol. Equip. 1991, 5, 32–36.
- Povolo, C.; Avolio, R.; Doria, E.; Marra, A. Development and validation of an analytical method to ensure quality requirements of hydrolysed proteins intended for agricultural use as biostimulants. Talanta Open 2022, 5, 100082.
- Jensen, C.; Dale, H.F.; Hausken, T.; Hatlebakk, J.G.; Bronstad, I.; Lied, G.A.; Hoff, D.A.L. Supplementation with low doses of a cod protein hydrolysate on glucose regulation and lipid metabolism in adults with metabolic syndrome: A randomized, double-blind study. Nutrients 2020, 12, 1991.
- Sarabandi, K.; Gharehbeglou, P.; Jafari, S.M. Spray-drying encapsulation of protein hydrolysates and bioactive peptides: Opportunities and challenges. Dry. Technol. 2020, 38, 577–595.
- Balan, D.; Luta, G.; Stanca, M.; Jerca, O.; Niculescu, M.; Gaidau, C.; Jurcoane, S.; Mihalcea, A. Effect of protein gel treatments on biometric and biochemical attributes of tomato seedlings in greenhouse condition. Agriculture 2023, 13, 54.
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8, 433.
- Ertani, A.; Francioso, O.; Ferrari, E.; Schiavon, M.; Nardi, S. Spectroscopic-chemical fingerprint and biostimulant activity of a protein-based product in solid form. Molecules 2018, 23, 1031.
- Dash, P.; Ghosh, G. Amino acid profiling and antimicrobial activity of Cucurbita moschata and Lagenaria siceraria seed protein hydrolysates. Nat. Prod. Res. 2018, 32, 2050–2053.
- Schmidt, G.J.; Olson, D.C.; Slavin, W. Amino acid profiling of protein hydrolysates using liquid chromatography and fluorescence detection. J. Liquid Chromatogr. 1979, 2, 1031–1045.
- Phetchthumrongchai, T.; Tachapuripunya, V.; Chintong, S.; Roytrakul, S.; E-kobon, T.; Klaypradit, W. Properties of protein hydrolysates and bioinformatics prediction of peptides derived from thermal and enzymatic process of Skipjack tuna (Katsuwonus pelamis) roe. Fishes 2022, 7, 255.
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Dinesh Kumar, B. Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg). J. Food Sci. Technol. 2015, 52, 5817–5825.
- Bilenky, M.; Nair, A. Biostimulants combined with water soluble fertilizer had little effect on transplant growth and pepper yield grown under greenhouse conditions. Int. J. Veg. Sci. 2023, 29, 25–39.
- Ghorbel-Bellaaj, O.; Jellouli, K.; Maalej, H. Shrimp processing by-products protein hydrolysates: Evaluation of antioxiant activity and application in biomass and proteases production. Biocatal. Biotransform. 2017, 35, 287–297.
- Li, H.; Aluko, R.E. Structural modulation of calmodulin and calmodulin-dependent protein kinase II by pea protein hydrolysates. Int. J. Food Sci. Nutr. 2006, 57, 178–189.
- Paradikovic, N.; Vinkovic, T.; Vinkovic Vrcek, I.; Zuntar, I.; Bojic, M.; Medic-Saric, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152.
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131.
- Paradikovic, N.; Teklic, T.; Zelikovic, S.; Lisjak, M.; Spoljarevic, M. Biostimulants research in some horticultural plant species- A review. Food Energy Secur. 2019, 8, e00162.
- Wilson, H.T.; Amirkhani, M.; Taylor, A.G. Evaluation of gelatin as a biostimulant seed treatment to improve plant performance. Front. Plant Sci. 2018, 9, 1006.
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Anthoxidants Redox Signal. 2013, 19, 998–1011.
- Vranova, V.; Rejsek, K.; Skene, K.R.; Formanek, P. Non-protein amino acids: Plant, soil, and ecosystem interactions. Plant Soil 2011, 342, 31–48.
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216.
- Flores, T.; Todd, C.D.; Tovar-Mendez, A.; Dhanoa, P.K.; Correa-Aragunde, N.; Hoyos, M.E.; Brownfield, D.M.; Mullen, R.T.; Lamattina, L.; Polacco, J.C. Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiol. 2008, 147, 1936–1946.
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726.
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzalka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant 2013, 35, 985–999.
- Rouphael, Y.; Colla, G.; Hoagland, L.; Giordano, M.; El-Nakhel, C.; Cardarelli, M. Vegetal-protein hydrolysates based microgranule enhances growth, mineral content, and quality traits of vegetable transplants. Sci. Hortic. 2021, 290, 110554.
- Colantoni, A.; Recchia, L.; Bernaberi, G.; Cardarelli, M.; Rouphael, Y.; Colla, G. Analyzing the environmental impact of chemically-produced protein hydrolysate from leather waste vs. enzymatically-produced protein hydrolysate from legume grains. Agriculture 2017, 7, 62.
- Porterfield, D.M. Environmental sensing and directional growth of plant roots. In Plant Roots: The Hidden Half, 4th ed.; CRC Press: Boca Raton, FL, USA, 2002.
- Trevisan, S.; Manoli, A.; Ravazzolo, L.; Franceschi, C.; Quaggiotti, S. mRNA-sequencing analysis reveals transcriptional changes in root of maize seedings treated with two increasing concentrations of a new biostimulant. J. Agric. Food Chem. 2017, 65, 9956–9969.
- Kisvarga, S.; Farkas, D.; Boronkay, G.; Nemenyi, A.; Orloci, L. Effects of biostimulants in horticulture, with emphasis on ornamental plant production. Agronomy 2022, 12, 1043.
- Tanou, G.; Ziogas, V.; Molassiotis, A. Foliar nutrition, biostimulants and prime-like dynamics in fruit tree physiology: New insights on an old topic. Front. Plant Sci. 2017, 8, 75.
- Malecange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant properties of protein hydrolysates: Recent advances and future challenges. Int. J. Mol. Sci. 2023, 24, 9714.
- Bajpai, M.; Pande, A.; Tewari, S.K.; Prakash, D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int. J. Food Sci. Nutr. 2005, 56, 287–291.
- Owen-Going, T.N.; Beninger, C.W.; Sutton, J.C.; Hall, J.C. Accumulation of phenolic compounds in plants and nutrient solution of hydroponically grown peppers inoculated with Pythium aphanidermatum. Can. J. Plant Pathol. 2008, 30, 214–225.
- Prakash, D.; Suri, S.; Upadhyay, G.; Singh, B.N. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 2007, 58, 18–28.
- Wong, J.Y.; Matanjun, P.; Ooi, Y.B.H.; Chia, K.F. Characterization of phenolic compounds, carotenoids, vitamins and antioxidant activities of selected Malaysian wild edible plants. Int. J. Food Sci. Nutr. 2013, 5, 621–631.
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communications during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719.
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; Volume 661, pp. 23–67.
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 3242.
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504.
- Takshak, S.; Agrawal, S.B. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol. B Biol. 2019, 193, 51–88.
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368.
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585.
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343.
- Hajiboland, R.; Farhanghi, F. Remobilization of boron, photosynthesis, phenolic metabolism and anti-oxidant defense capacity in boron-deficient turnip (Brassica rapa L.) plants. Soil Sci. Plant Nutr. 2010, 56, 427–437.
- Schoneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Addit. Contamin. 2018, 35, 2455–2470.
- Mohamed, M.S.M.; Saleh, A.M.; Abdel-Farid, I.B.; El-Naggar, S.A. Growth, hydrolases and ultrastructure of Fusarium oxysporum as affected by phenolic rich extracts from several xerophytic plants. Pestic. Biochem. Physiol. 2017, 141, 57–64.
- Lwalaba, J.L.W.; Zvobgo, G.; Mwamba, T.M.; Louis, L.T.; Fu, L.; Kirika, B.A.; Tshibangu, A.K.; Adil, M.F.; Sehar, S.; Mukobo, R.P. High accumulation of phenolics and amino acids confers tolerance to the combined stress of cobalt and copper in barley (Hordeum vulgare). Plant Physiol. Biochem. 2020, 155, 927–937.
- Silva, G.H.; Souza, J.A.R.D.; Macedo, W.R.; Pinto, F.G. Tyrosol, a phenolic compound from Phomopsis sp., is a potential biostimulant in soybean seed treatment. Phytochem. Lett. 2021, 43, 40–44.
- Masondo, N.A.; Aremu, A.O.; Rengasamy, K.R.R.; Amoo, S.O.; Gruz, J.; Subrtova, M.; Dolezal, K.; Van Staden, J. Growth and phytochemical response in Eucomis autumnalis (Mill.) Chitt. Treated with phenolic biostimulants from brown alga, Ecklonia maxima. S. Afr. J. Bot. 2015, 98, 211.
- Chen, S.; Lin, R.; Lu, H.; Wang, Q.; Yang, J.; Liu, J.; Yan, C. Effects of phenolic acids on free radical scavenging and heavy metal bioavailability in Kandelia obovata under cadmium and zinc stress. Chemosphere 2020, 249, 126341.
- Yahia, Y.; Bagues, M.; Zaghdoud, C.; Al-Amri, S.M.; Nagaz, K.; Guerfel, M. Phenolic profile, antioxidant capacity and antimicrobial activity of Calligonum arich L., desert endemic plant in Tunisia. S Afr. J. Bot. 2019, 124, 414–419.
- Cueto, J.D.; Kosinska-Cagnazzo, A.; Stefani, P.; Heritier, J.; Roch, G.; Oberhansli, T.; Audergon, J.-M.; Christen, D. Phenolic compounds identified in apricot branch tissues and their role in the control of Monilinia laxa growth. Sci. Hortic. 2021, 275, 109707.
- Jakubke, H.D.; Jeschkeit, H. Aminokwasy, Peptydy, Bialka, 2nd ed.; Polskie Wydawnictwo Naukowe: Warsaw, Poland, 1989.
- Shahrajabian, M.H.; Khoshkharam, M.; Sun, W.; Cheng, Q. Germination and seedlings growth of corn (Zea mays L.) to allelopathic effects of rice (Oryza sativa L.). Trop. Plant Res. 2019, 6, 152–156.
- Del Bubba, M.; Ancillotti, C.; Checchini, L.; Ciofi, L.; Fibbi, D.; Gonnelli, C.; Mosti, S. Chromium accumulation and changes in plant growth, selected phenolics and sugars of wild type and genetically modified Nicotiana langsdorfii. J. Hazard. Mater. 2013, 262, 394–403.
- Kucinska, J.K.; Magnucka, E.G.; Oksinska, M.P.; Pietr, S.J. Bioefficacy of hen feather keratin hydrolysate and compost on vegetable plant growth. Compost Sci. Utiliz. 2014, 22, 179–187.
- Tejada, M.; Rodriguez-Morgado, B.; Paneque, P.; Parrado, J. Effects of foliar fertilization of a biostimulant obtained from chicken feathers on maize yield. Eur. J. Agron. 2018, 96, 54–59.
- Wong, F.-C.; Chai, T.-T. Bioactive peptides and protein hydrolysates as lipoxygenase inhibitors. Biology 2023, 12, 917.
