Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
In the quest for sustainable energy solutions and environmental protection, the management of end-of-life (EoL) batteries has emerged as a critical issue. Batteries, especially lithium-ion batteries (LIBs), power a wide range of devices and are central to modern life. As society’s reliance on batteries grows, there is an urgent need for sustainable battery recycling methods that can efficiently recover valuable materials, minimize environmental impact, and support the circular economy.
- recycling
- electrode
- electrolyte
- separator
- spent batteries
- lithium-ion batteries (LIBs)
1. Introduction
The circular economy of LIBs consists of different phases, starting with the extraction of raw materials, through production and the use phase for disposal, reprocessing, recycling and the subsequent substitution of primary raw materials. In particular, the phases from disposal and collection to metallurgical processing into secondary raw materials play a central role in the recycling of the LIBs. In general, the end-of-life processes for LIBs contain most or all of the following process steps:
The following sections present the current state-of-the-art in sorting, processing and recycling LIBs. The dismantling and discharging steps occur mainly in the recycling of LIBs from electric mobility [6]. Depending on the recycling route, thermal deactivation of LIB cells is also required [9].
2. Basics of Lithium-Ion Batteries
LIB cells consist of two electrodes (anode and cathode), an electrolyte and a separator. The operation of an LIB cell is based on the principle of reversible lithium-ion intercalation and de-intercalation during charging and discharging [17]. Between the electrodes is an ion-conducting electrolyte containing a dissociated lithium-conducting salt, commonly used is LiPF6. Other components of the electrolyte are organic carbonates, such as diethyl carbonate, dimethyl carbonate and others [17]. A porous plastic film separates the two electrodes from each other and acts as a membrane for lithium ions [18]. The cathode of commercial LIB cells usually consists of a compound that can accept lithium ions, such as transition metal oxides or polyanion compounds [19]. The most commonly used transition metal oxides are the following [20][21][22][23][24][25][26]:
-
Lithium cobalt oxide (LiCoO2, LCO),
-
Lithium manganese oxide (LiMn2O4, LMO),
-
Lithium nickel cobalt manganese oxide (LiNiXMnYCoZO2, NMC),
-
Lithium nickel aluminum oxide (LiNi0.8Co0.15Al0.05O2, NCA).
-
Lithium iron phosphate (LiFePO4, LFP),
-
Lithium manganese phosphate (LiMnPO4, LMP),
-
Lithium cobalt phosphate (LiCoPO4, LCP).
Aluminum foils are used as the current arrester for cathodes. In commercial use, most anodes use graphite as the anode active material [30][31][32] and a thin copper foil as the current arrester.
LIB are designed and built as individual cells. These cells are manufactured in various formats, and a distinction is made between button, cylindrical, prismatic and pouch cells [27][33]. Cylindrical cells are manufactured in different layers of anode, separator and cathode, which are rolled around a pin to a so-called jelly roll [34]. The jelly roll is further integrated into a steel shell, which is filled with the electrolyte, closed and welded [34]. Pouch cells are also manufactured with different layers of anode, separator and cathode sheets. These are stacked on top of each other, inserted into the pouch foil, a very thin aluminum-polymer compound, before being filled with the electrolyte and closed [34]. For cylindrical cells, the 18650 and 21700 cell formats, in particular, have become established [27]. The 4680 cell format is being discussed in particular by American car manufacturer Tesla and will be used there in the future [35][36]. The 18650 cell format is used for portable batteries in the consumer sector. The dimensions of this format are standardized by the American National Standards Institute in the ANSI C18.1M standard [37], while the dimensions of the prismatic and pouch cell formats for automotive applications are standardized by DIN SPEC 91252 [33]. Pouch cells used in the consumer sector for products such as laptops, tablets and phones are currently not subject to any standardization. Depending on the application, several cells can be connected in series and/or in parallel in a module [18]. Modules use a battery management system (BMS) to manage individual cells. The BMS determines the cell voltage and temperatures, monitors the current, and allows the battery system to be switched on and off [18]. For EV batteries, several modules are connected to a battery pack. Current trends also pursue the “cell-to-pack” approach, in which many cells are directly interconnected into a battery pack [38].
3. Collection and Sorting of Spent Batteries
Since the Battery Directive came into force in 1998, manufacturers and importers of batteries have been required to take back and recycle spent batteries and accumulators free of charge. The legislator provides so-called collection schemes for the return and proper recycling [39]. There are various collection schemes in Germany and other European countries, such as the following:
In Germany, spent batteries as well as waste electrical appliances must be taken back by every retailer according to § 9 BattG and § 17(1) 2. ElektroG2 [44][45]. For the individual cells and battery packs, there are disposal boxes in which the consumer can separate the used batteries into LIBs and others. Since a mixed waste battery fraction with different chemical compositions cannot be recycled [39][46] and a sorted return by the consumer cannot be guaranteed, sorting based on the chemical composition of the waste batteries is necessary.
Sorting spent batteries according to their chemical composition can be done in different ways. A distinction is made between mechanical, automatic and manual sorting [39]. Mechanical sorting of individual, general purpose portable batteries is carried out, for example, by sieving, where the spent batteries are sorted according to their size [47]. In manual (or optical) sorting, batteries are sorted by humans based on visual characteristics, such as type designation or manufacturer. Typical sorting speeds are around 300 kg of batteries per person per hour [39]. Currently, every battery sorting facility in Europe performs (partially) the manual sorting of battery cells or battery packs. Automatic sorting of spent batteries according to their chemical composition (Li-ion, NiCd, NiMH, etc.) can be done by sensor-based sorting. In practice, XRT sorting [47], VIS sorting and sorting by weight sensors are used [48]. Currently, sorting is only done based on the battery type but not further by CAM. Figure 1 shows state-of-the-art sorting concepts for EoL batteries. In some research concepts, LIB cells have been further sorted into cobalt-rich and cobalt-poor fractions, but good data on the results have not been published [49].
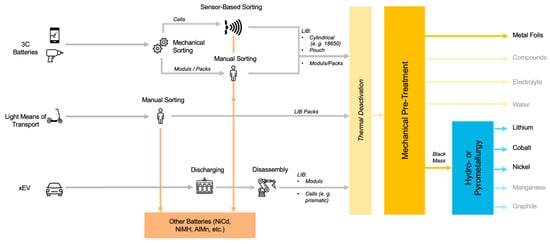
Figure 1. State of the Art Battery Sorting Concepts (own illustration).
4. Mechanical Treatment of Spent Lithium-Ion Batteries
After collection and sorting, LIBs are pre-treated depending on the subsequent mechanical and metallurgical processing steps. These include the processes of deep discharging [50][51][52][53], dismantling [54] and thermal pretreatment [9]. Due to its mostly manual execution, the process of deep unloading is currently only carried out for EV-LIB [55]. 3C-LIBs are largely thermally deactivated [9] or crushed in a wet environment [56], in which case deep discharge is not necessary. Current developments in automated deep discharge are also carried out exclusively for EV applications [57]. LIBs in module, pack or cell format represent a composite material consisting of many different components. To separate them for subsequent material recycling, the various plug-in, welded and bonded joints must be separated from each other. Size reduction has been a proven method in other waste streams, including LIB recycling. Depending on the pre-treatment measures implemented for the LIB, the following forms of mechanical pulping are used:
-
Size reduction after thermal deactivation [58];
Depending on the pre-treatment and mechanical shredding, the (residual) electrolyte components are removed from the shredder output. In the case of thermal deactivation of the cells by means of pyrolysis, the electrolyte components are no longer present in the material stream prior to shredding; thus, electrolyte removal is not necessary in this case. Various processes have been investigated and developed for electrolyte removal after mechanical comminution, such as extraction by thermal drying [62], solvent extraction [63] and extraction by supercritical CO2 [64]. For further processing of the dry, non-organic material stream, the various materials need to be separated from each other. As in the processing of other waste streams, the aim is to sort the different materials as clean as possible. LIBs consist of many different components, some of which have very fine and complex structures and are therefore not easy to separate. An important sub-goal of material separation is the separation of the active materials for further metallurgical processing. Due to the small particle size of the active materials, screening is used [65]. This is typically used immediately after drying or (re)crushing. The separation and recovery of graphite from the active material mixture is performed by selective flotation [66]. This common process has been patented by Retriev Technologies [67] and the Warner Babcock Institute for Green Chemistry [68], among others. For the separation of ferromagnetic materials, a magnetic separator is usually used [69], as in other mechanical processing plants. The further separation of the conductor foils (consisting of aluminum and copper) from the less economically valuable separator foil, made from materials such as polyethylene or polypropylene, can be performed using different technologies. On the one hand, zig zag separators are used to exploit the density differences between the plastic and metal components [8]. In a two-stage separation process, the heavy metal components, such as housing parts, and then the light metal components, such as aluminum and copper foils, can be separated from the plastics. In wet mechanical processes, float-sink separation is another form of density separation to sort the metal and plastic components [56]. An alternative is separation with an eddy current separator. Here, the non-ferrous metals are induced by a rotating electromagnet and are thus separated from the non-magnetizable particles, such as the separator films [31].
5. Metallurgical Recycling
Due to the physical and chemical properties of the active materials, such as the small grain size, mechanical processes are not able to further separate the black mass by material components. Metallurgical processes can be used for these processing steps. Pyrometallurgy includes all processes aimed at recovering or refining metals at elevated temperatures [70]. In practice, either an electric arc furnace (EAF) or a shaft furnace (SF) are used to recycle EoL-LIB. These processes are part of extractive pyrometallurgy. Different pre-treatment processes are required for the pyrometallurgical processing of LIBs in EAF and SF. While only the black mass can be processed in EAF, whole LIB cells can also be recycled in SF [70]. Both processes use controlled reduction as the separation process so that the elements nickel, cobalt and copper end up in the metal phase and lithium, manganese, titanium, silicon, aluminum and iron in the slag phase. In this way, recycling efficiencies of around 60 percent can be achieved [70].
Hydrometallurgical recycling of LIBs primarily refers to the recovery of individual valuable metals from cathode active materials. This usually involves a combination of leaching and subsequent extraction processes. Leaching is a key step in the recovery process. The aim of leaching is to bring the metals of the cathode active materials into solution as ions. These can then be recovered via various extraction processes [71]. In hydrometallurgical recycling, a distinction is made between two feed streams depending on the pre-treatment. In the case of pyrometallurgical or thermal pre-treatment of the LIB or active mass, the alloy of copper, nickel, cobalt and iron is brought into solution by leaching. If no thermal pre-treatment is used, e.g., in the case of mechanical processing in an inert atmosphere [72], the metallic components are leached and the insoluble components such as graphite and, if applicable, binder are filtered off [73]. After leaching, the solution is first cleaned by hydroxide precipitation. In this process, copper and aluminum impurities are precipitated with the addition of, e.g., NaOH [74]. For the subsequent extraction of precious metals from the solution, either further precipitation steps or solvent extraction are used [75]. Chemical precipitation of precious metals results in the formation of insoluble compounds through the addition of suitable precipitants [76][77][78][79][80][81][82]. Solvent extraction is a process in which a two-phase system, usually consisting of an organic and an aqueous phase, is introduced. Here, separation can be achieved by the unequal distribution of the two phases, where solvent extraction agents with high selectivity are used after leaching to separate specific transition metals from the leach solution [82][83][84]. The lithium then remains in the solution and can be precipitated, e.g., as lithium carbonate, by adding sodium carbonate [74].
In addition to pyrometallurgy and hydrometallurgy, the direct recycling of the active material is a third alternative. This process has been developed for the reuse of cathode active material from LIB recycling in the production of new LIBs. The process basically consists of two process steps: the recovery of electrode material from LIBs and the subsequent rejuvenation of the recycled electrode material [84]. In this context, the process of re-lithiation by the hydrothermal method, the electrochemical method and the direct calcination method has been extensively researched [85][86]. Success on a laboratory scale has already been achieved in various studies, but industrial implementation has not yet taken place.
This entry is adapted from the peer-reviewed paper 10.3390/met14020151
References
- Oliveira Neto, G.C.; Ruiz, M.S.; Correia, A.J.C.; Mendes, H.M.R. Environmental advantages of the reverse logistics: A case study in the batteries collection in Brazil. Production 2018, 28, e20170098.
- Nigl, T.; Schwarz, T.E.; Walch, C.; Baldauf, M.; Rutrecht, B.; Pomberger, R. Characterisation and material flow analysis of end-of-life portable batteries and lithium-based batteries in different waste streams in Austria. Waste Manag. Res. 2020, 38, 649–659.
- Ponce-Cueto, E.; Manteca, J.Á.G.; Carrasco-Gallego, R. Reverse Logistics for Used Portable Batteries in Spain: An Analytical Proposal for Collecting Batteries; Springer: Berlin/Heidelberg, Germany, 2011; pp. 593–604.
- Rogulski, Z.; Czerwiński, A. Used batteries collection and recycling in Poland. J. Power Sources 2006, 159, 454–458.
- Terazono, A.; Oguchi, M.; Iino, S.; Mogi, S. Battery collection in municipal waste management in Japan: Challenges for hazardous substance control and safety. Waste Manag. 2015, 39, 246–257.
- Zorn, M.; Ionescu, C.; Klohs, D.; Zähl, K.; Kisseler, N.; Daldrup, A.; Hams, S.; Zheng, Y.; Offermanns, C.; Flamme, S.; et al. An Approach for Automated Disassembly of Lithium-Ion Battery Packs and High-Quality Recycling Using Computer Vision, Labeling, and Material Characterization. Recycling 2022, 7, 48.
- Wegener, K.; Andrew, S.; Raatz, A.; Dröder, K.; Herrmann, C. Disassembly of Electric Vehicle Batteries Using the Example of the Audi Q5 Hybrid System. Procedia CIRP 2014, 23, 155–160.
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311.
- Arnberger, A. Entwicklung Eines Ganzheitlichen Recyclingkonzeptes für Traktionsbatterien Basierend auf Lithium-Ionen-Batterien. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 2016.
- Vest, M. Weiterentwicklung des Pyrometallurgischen IME Recyclingverfahrens für Li-Ionen Batterien von Elektrofahrzeugen. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2016.
- Georgi-Maschler, T. Entwicklung Eines Recyclingverfahrens für Portable Li-Ion-Gerätebatterien. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2011.
- Arnberger, A.; Coskun, E.; Rutrecht, B. Recycling von Lithium-Ionen-Batterien. In Recycling und Rohstoffe; Thiel, S., Thomé-Kozmiensky, E., Goldmann, D., Eds.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2018; pp. 583–599. ISBN 978-3-944310-40-4.
- Zhang, T.; He, Y.; Ge, L.; Fu, R.; Zhang, X.; Huang, Y. Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries. J. Power Sources 2013, 240, 766–771.
- Diekmann, J.; Sander, S.; Sellin, G.; Petermann, M.; Kwade, A. Crushing of Battery Modules and Cells. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 127–138. ISBN 978-3-319-70571-2.
- Pagliaro, M.; Meneguzzo, F. Recycling of Lithium Batteries. In Sustainable Separation Engineering: Materials, Techniques and Process Development, 1st ed.; Szekely, G., Zhao, D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 591–603. ISBN 978-1-119-74008-7.
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156.
- Korthauer, R. (Ed.) Handbuch Lithium-Ionen-Batterien, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-30652-5.
- Leuthner, S. Übersicht zu Lithium-Ionen-Batterien. In Handbuch Lithium-Ionen-Batterien, 1st ed.; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 13–19. ISBN 978-3-642-30652-5.
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264.
- Dorrmann, L.; Sann-Ferro, K.; Heininger, P.; Mähliß, J. Kompendium: Li-Ionen-Batterien: Grundlagen, Merkmale, Gesetze und Normen. Available online: https://www.dke.de/resource/blob/933404/fa7a24099c84ef613d8e7afd2c860a39/kompendium-li-ionen-batterien-data.pdf (accessed on 1 May 2023).
- Kalyani, P.; Kalaiselvi, N. Various Aspects of LiNiO2 Chemistry: A Review. Sci. Technol. Adv. Mater. 2005, 6, 689–703.
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; et al. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 2013, 7, 760–767.
- Yabuuchi, N.; Ohzuku, T. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J. Power Sources 2003, 119–121, 171–174.
- Doughty, D.H.; Roth, E.P. A General Discussion of Li Ion Battery Safety. Electrochem. Soc. Interface 2012, 21, 37–44.
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28.
- Li, W.; Lee, S.; Manthiram, A. High-Nickel NMA: A Cobalt-Free Alternative to NMC and NCA Cathodes for Lithium-Ion Batteries. Adv. Mater. 2020, 32, e2002718.
- Link, S.; Neef, C.; Wicke, T.; Hettesheimer, T.; Diehl, M.; Krätzig, O.; Degen, F.; Klein, F.; Fanz, P.; Burgard, M.; et al. Development Perspectives for Lithium-Ion Battery Cell Formats; Fraunhofer: Karlsruhe, Germany, 2022; Available online: https://www.isi.fraunhofer.de/content/dam/isi/dokumente/cct/2022/Development_perspectives_for_lithium-ion_battery_cell_formats_Fraunhofer_2022.pdf (accessed on 20 April 2023).
- Song, J.; Li, B.; Chen, Y.; Zuo, Y.; Ning, F.; Shang, H.; Feng, G.; Liu, N.; Shen, C.; Ai, X.; et al. A High-Performance Li-Mn-O Li-rich Cathode Material with Rhombohedral Symmetry via Intralayer Li/Mn Disordering. Adv. Mater. 2020, 32, e2000190.
- Zheng, J.; Myeong, S.; Cho, W.; Yan, P.; Xiao, J.; Wang, C.; Cho, J.; Zhang, J.-G. Li- and Mn-Rich Cathode Materials: Challenges to Commercialization. Adv. Energy Mater. 2017, 7, 1601284.
- Wurm, C.; Öttinger, O.; Wittkämper, S.; Zauter, R.; Vuorilehto, K. Anodenmaterialien für Lithium-Ionen-Batterien. In Handbuch Lithium-Ionen-Batterien, 1st ed.; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 45–60. ISBN 978-3-642-30652-5.
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170.
- Wu, Y. Modified natural graphite as anode material for lithium-ion batteries. J. Power Sources 2002, 111, 229–334.
- Hettesmeier, T.; Thielmann, A.; Neef, C.; Möller, K.-C.; Wolter, M.; Lorentz, V.; Gepp, M.; Wenger, M.; Prill, T.; Zausch, J.; et al. Entwicklungsperspektiven für Zellformate von Lithium-Ionen-Batterien in der Elektromobilität; Fraunhofer: Pfinztal, Germany, 2017; Available online: https://publica.fraunhofer.de/entities/publication/0ca7ef53-3b51-4467-ad20-bdef36958520/details (accessed on 4 May 2023).
- Heimes, H.; Kampker, A.; Wennemar, S.; Plocher, L.; Bockey, G.; Michaelis, S.; Schütrumpf, J. Production Process of a Lithium-Ion Battery Cell. Available online: https://www.pem.rwth-aachen.de/global/show_document.asp?id=aaaaaaaabyilawq (accessed on 18 December 2023).
- Tesla. Battery Day Presentation Deck. 2020. Available online: https://tesla-share.thron.com/content/?id=96ea71cf-8fda-4648-a62c-753af436c3b6&pkey=S1dbei4 (accessed on 10 May 2023).
- Frank, A.; Sturm, J.; Steinhardt, M.; Rheinfeld, A.; Jossen, A. Impact of Current Collector Design and Cooling Topology on Fast Charging of Cylindrical Lithium-Ion Batteries. ECS Adv. 2022, 1, 40502.
- American National Standards Institute. ANSI C18.1M; American National Standard for Portable Primary Cells and Batteries with Aqueous Electrolyte: General and Specifications. National Electrical Manufacturers Association: Rosslyn, VA, USA, 2021. Available online: https://www.nema.org/docs/default-source/standards-document-library/ansi-c18.1m-part-1-2021-contents-and-scope03ee42fa-4df1-4659-af17-1152feebb97f.pdf?sfvrsn=a1875742_3 (accessed on 21 December 2023).
- Gerlitz, E.; Botzem, D.; Weinmann, H.; Ruhland, J.; Fleischer, J. Cell-to-Pack-Technologie für Li-Ionen-Batterien. Z. Für Wirtsch. Fabr. 2021, 116, 689–694.
- Sziegoleit, H. Sortierung von Gerätebatterien. In Recycling und Rohstoffe, 6th ed.; Thomé-Kozmiensky, K.J., Goldmann, D., Eds.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2013; pp. 495–504. ISBN 978-3-935317-97-9.
- Recycling Magazin. GRS und Saubermacher Digitalisieren Rücknahmesysteme. Available online: https://www.recyclingmagazin.de/2021/12/22/grs-und-saubermacher-digitalisieren-ruecknahmesysteme/ (accessed on 22 May 2023).
- REBAT. Internetauftritt der Firma. Available online: https://rebat.de/ (accessed on 22 May 2023).
- Landbell Group. Internetauftritt der Firma. Available online: https://www.landbell.de/ds-entsorgung/ (accessed on 22 May 2023).
- Stibat, B.V. Internetauftritt der Firma. Available online: https://www.stibat.nl/ (accessed on 22 May 2023).
- Bundesministerium der Justiz. Gesetz über das Inverkehrbringen, die Rücknahme und die Umweltverträgliche Entsorgung von Batterien und Akkumulatoren: Batteriegesetz-BattG; Bundesministerium der Justiz: Berlin/Heidelberg, Germany, 2009.
- Bundesministerium der Justiz. Gesetz über das Inverkehrbringen, die Rücknahme und die Umweltverträgliche Entsorgung von Elektro- und Elektronikgeräten: Elektro- und Elektronikgerätegesetz-ElektroG; Bundesministerium der Justiz: Berlin/Heidelberg, Germany, 2015.
- Samarukha, I. Recycling strategies for End-of-Life Li-ion Batteries from Heavy Electric Vehicles. Master’s Thesis, Königliche Technische Hochschule, Stockholm, Sweden, 2020.
- Tomra Sorting GmbH. Relux Installiert Tomras X-Tract zur Erfolgreichen Batteriesortierung. Available online: https://languagesites.tomra.com/de-de/sorting/recycling/recycling-news/2021/relux-installs-tomra-xtract-successful-battery-sorting (accessed on 13 March 2023).
- Bebat, N.V. Bebat—Behind the Factory. Available online: https://www.youtube.com/watch?v=Hd2MBmurPJM (accessed on 13 March 2023).
- Sletsgaard, J.; Hald Pedersen, N. Øget Ressourcegenvinding ved Forbedret Karakterisering af Affaldsbatterier: Miljøteknologisk Udviklings- og Demonstrationsprogram 2012; Miljøprojekt: Kopenhagen, Denmark, 2014.
- Blank, T.; Badeda, J.; Kowal, J.; Sauer, D.U. Deep discharge behavior of lead-acid batteries and modeling of stationary battery energy storage systems. In Proceedings of the Intelec 2012 IEEE International Telecommunications Energy Conference, Scottsdale, AZ, USA, 30 September–4 October 2012; pp. 1–4, ISBN 978-1-4673-1000-0.
- Langner, T.; Sieber, T.; Acker, J. Studies on the deposition of copper in lithium-ion batteries during the deep discharge process. Sci. Rep. 2021, 11, 6316.
- Ahrens, J. Rechargeable Battery Discharge Device for Discharging Rechargeable Batteries, and Method for Discharging a Plurality of Rechargeable Batteries. U.S. Patent Application No. 18/005,096, 12 July 2021.
- Hanisch, C.; Bußmann, T. Verfahren zum Wiederverwerten von Akkumulatoren und Akkumulator-Entladevorrichtung. DE102020118418A1, 18 March 2019.
- Gerbers, R.; Wegener, K.; Dietrich, F. Safe, Flexible and Productive Human-Robot-Collaboration for Disassembly of Lithium-Ion Batteries. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 99–126. ISBN 978-3-319-70571-2.
- Duesenfeld GmbH. Company Website. Available online: https://www.duesenfeld.com/ (accessed on 15 October 2023).
- Petzold, M. Nassmechanische Zerkleinerung und Aufbereitung von Lithium-Ionen-Batterien aus Elektrofahrzeugen zur optimierten Lithium-Rückgewinnung. In 12. Wissenschaftskongress Abfall- und Ressourcenwirtschaft am 9. und 10. März 2023 an der Technischen Universität Hamburg, Hamburg, 09.03.—10.03.2023; Deutsche Gesellschaft für Abfallwirtschaft, e.V., Ed.; Innsbruck University Press: Innsbruck, Austria, 2023; pp. 19–24. ISBN 978-3-99106-095-6.
- Christmann, D. Batterierecycling: Bosch Entwickelt Europas Erste vollautomatisierte Anlage zur Batterieentladung. Available online: https://www.bosch-presse.de/pressportal/de/de/batterierecycling-bosch-entwickelt-europas-erste-vollautomatisierte-anlage-zur-batteriedemontage-252928.html (accessed on 29 May 2023).
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622.
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86.
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68.
- Petzold, M.; Flamme, S.; Hams, S. Nassmechanische Aufbereitung von Lithium-Ionen Batterien. Müll und Abfall, 8 December 2023.
- Haas, P.; Pfeifer, S.; Müller, J.; Bradtmöller, C.; Scholl, S. Separation of the Electrolyte—Solvent Extraction. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 155–176. ISBN 978-3-319-70571-2.
- Stehmann, F.; Bradtmöller, C.; Scholl, S. Separation of the Electrolyte—Thermal Drying. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 139–154. ISBN 978-3-319-70571-2.
- Rothermel, S.; Grützke, M.; Mönnighoff, X.; Winter, M.; Nowak, S. Electrolyte Extraction—Sub and Supercritical CO2. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 177–186. ISBN 978-3-319-70571-2.
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium-Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416.
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801.
- Novis Smith, W.; Swoffer, S. Recovery of Lithium-Ion Batteries. U.S. Patent 861475 B1, 18 June 2013.
- Poe, S.L.; Paradise, C.L.; Muollo, L.R.; Pal, R.; Warner, J.C.; Korzenski, M.B. Method for the Recovery of Lithium Cobalt Oxide from Lithium-Ion Batteries. U.S. Patent 9972830, 19 June 2012.
- Vieceli, N.; Nogueira, C.A.; Guimarães, C.; Pereira, M.F.C.; Durão, F.O.; Margarido, F. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 2018, 71, 350–361.
- Peters, L.; Friedrich, B. Proven Methods for Recovery of Lithium from Spent Batteries; DERA Workshop Lithium: Berlin, Germany, 2017.
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627.
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2017, 164, A6184–A6191.
- Fraunhofer-Institut für System- und Innovationsforschung ISI. Recycling von Lithium-Ionen- Batterien: Chancen und Herausforderungen für den Maschinen- und Anlagenbau; Fraunhofer: Karlsruhe, Germany, 2021.
- Wang, H.; Vest, M.; Friedrich, B. Hydrometallurgical processing of Li-Ion battery scrap from electric vehicles. In Proceedings of the European Metallurgical Conference 2011, Düsseldorf, Germany, 22–24 June 2011; pp. 1033–1052.
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium-ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293.
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A closed loop process for recycling spent lithium-ion batteries. J. Power Sources 2014, 262, 255–262.
- Zhu, S.; He, W.; Li, G.; Zhou, X.; Zhang, X.; Huang, J. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans. Nonferrous Met. Soc. China 2012, 22, 2274–2281.
- Contestabile, M.; Panero, S.; Scrosati, B. A laboratory-scale lithium-ion battery recycling process. J. Power Sources 2001, 92, 65–69.
- Pegoretti, V.; Dixini, P.; Smecellato, P.C.; Biaggio, S.R.; Freitas, M. Thermal synthesis, characterization and electrochemical study of high-temperature (HT) LiCoO2 obtained from Co(OH)2 recycled of spent lithium-ion batteries. Mater. Res. Bull. 2017, 86, 5–9.
- Yang, Y.; Zheng, X.; Cao, H.; Zhao, C.; Lin, X.; Ning, P.; Zhang, Y.; Jin, W.; Sun, Z. A Closed-Loop Process for Selective Metal Recovery from Spent Lithium Iron Phosphate Batteries through Mechanochemical Activation. ACS Sustain. Chem. Eng. 2017, 5, 9972–9980.
- Chen, X.; Luo, C.; Zhang, J.; Kong, J.; Zhou, T. Sustainable Recovery of Metals from Spent Lithium-Ion Batteries: A Green Process. ACS Sustain. Chem. Eng. 2015, 3, 3104–3113.
- Nan, J.; Han, D.; Zuo, X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J. Power Sources 2005, 152, 278–284.
- Swain, B.; Jeong, J.; Lee, J.; Lee, G.-H. Separation of cobalt and lithium from mixed sulphate solution using Na-Cyanex 272. Hydrometallurgy 2006, 84, 130–138.
- Zhan, R.; Payne, T.; Leftwich, T.; Perrine, K.; Pan, L. De-agglomeration of cathode composites for direct recycling of Li-ion batteries. Waste Manag. 2020, 105, 39–48.
- Zhang, X.; Xue, Q.; Li, L.; Fan, E.; Wu, F.; Chen, R. Sustainable Recycling and Regeneration of Cathode Scraps from Industrial Production of Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 7041–7049.
- Ganter, M.J.; Landi, B.J.; Babbitt, C.W.; Anctil, A.; Gaustad, G. Cathode refunctionalization as a lithium ion battery recycling alternative. J. Power Sources 2014, 256, 274–280.
This entry is offline, you can click here to edit this entry!
