Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive hematologic cancer originating from the malignant transformation of plasmacytoid dendritic cell precursors. The exploration of combinations such as CD123-targeted immunotherapies with azacitidine and venetoclax is suggested to enhance antineoplastic responses and improve survival rates in BPDCN patients.
- BPDCN
- dendritic cells
- pDC
- cancer therapy
- immunotherapy
1. Introduction
The neoplastic proliferation of plasmacytoid dendritic cells (pDCs) is known to give rise to BPDCN, as well as to the clonal expansion of mature pDCs [1]. First identified as a unique category in 1994, BPDCN is a rare and aggressive hematologic cancer originating from the malignant transformation of the non-activated precursors of pDC. This malignancy represents less than 1% of all hematologic tumor cases [2,3]. Its primary impact is observed in older adults, commonly surfacing in the sixth decade of life. Significantly, there is a higher prevalence among males, and individuals of Caucasian descent are slightly more affected than those of other demographics. Beyond its epidemiological profile, BPDCN exhibits a set of unique clinical, morphological, and immunophenotypic features, setting it apart from other hematopoietic neoplasms [4]. The nomenclature evolved in 2016 when the World Health Organization (WHO) classified BPDCN as a distinct malignancy within myeloid neoplasms and acute leukemia [5,6]. While the specific developmental pathway of pDC malignancies remains a subject of debate, prevailing evidence supports that the malignant proliferation of pDC precursors is specifically involved in the pathogenesis of BPDCN [3,7,8]. To comprehend this, it is essential to delve into the classification of DCs and their functional subtypes.
1.1. Dendritic Cell Classification
Dendritic cells (DCs), originating from bone marrow, are a diverse group found in the circulation, lymphoid organs, and various tissues. Modern classification recognizes two principal functional subtypes: conventional DCs (cDCs) and pDCs [9,10]. In the bloodstream, there are cDCs that express CD11c, including cDC2 CD1c+ and cDC1 CD141+ cells. Plasmacytoid dendritic cells, characterized by CD123+ and CD303+ markers, are also present but do not express CD11c. Although pDCs make up a minor fraction of the DC population, they predominantly reside in the blood and lymphoid tissues [9,10,11]. These cells are renowned for their robust antiviral defense capabilities, primarily through the secretion of type I interferon (IFN). Despite their antiviral function, pDCs also play a role in immune tolerance, as evidenced by their ability to stimulate regulatory T cells (Tregs) and T cells producing interleukin (IL)-10, indicating a potentially dualistic role in immune response regulation [12,13,14].
1.2. Clinical Manifestations
The importance of accurately identifying and understanding BPDCN is emphasized to enhance clinical management and treatment strategies for this specific subset of hematologic malignancies. BPDCN patients typically initially show skin involvement, which can extend to affect bone marrow (BM), peripheral blood (PB), and lymph nodes (LN). Extra-cutaneous manifestations often appear at diagnosis, particularly in regional LNs, progressing to involve PB and BM as the disease advances [2,15]. This malignancy is characterized by rapid progression, frequent relapses, and poor overall survival (OS), highlighting the need for effective treatment strategies [16].
Historically, diagnosing and treating BPDCN have been challenging due to its rarity and the lack of standardized approaches. It predominantly affects the elderly, with a noticeable male prevalence, and most patients experience cytopenias, lymphadenopathy, and/or splenomegaly [2]. As leukemic presentation is infrequent, occurring in less than 1% of acute leukemia cases, only 10% of those initially presenting with isolated skin lesions progress rapidly to leukemia [2,17,18]. Interestingly, a minority of cases present with leukemia but without skin involvement [15]. Advances in immunophenotyping and molecular profiling have uncovered potential therapeutic targets.
1.3. Diagnosis of BPDCN
The accurate identification and classification of BPDCN require a combination of clinical expertise and precise diagnostic laboratory tests due to its clinical and biological heterogeneity. Immunophenotypic criteria, using either flow cytometry (FC) or immunohistochemistry (IHC), are the primary methods for identifying this neoplasm [19].
Flow cytometry is considered a crucial diagnostic tool for BPDCN due to its objectivity and quantitative precision. It has the ability to detect small populations of cells with abnormal phenotypes among normal leukocytes, which is particularly important for accurately diagnosing BPDCN. FC outperforms IHC in diagnosing BPDCN because it can simultaneously detect multiple antigens, including those that are not routinely examined via IHC. This heightened sensitivity is essential in overcoming the challenges associated with accurately diagnosing BPDCN. However, diagnosing BPDCN is challenging due to its immunophenotypic heterogeneity, which often leads to features overlapping other hematologic neoplasms such as NK/T cell leukemia/lymphoma, cutaneous T cell lymphoma, and acute myelomonocytic leukemia expressing CD4 or CD56 [20]. Moreover, the initial assumption that BPDCN derived from NK cells based on the absence of common lineage markers and the expression of CD4 and CD56 has been reconsidered. Further studies have revealed the intricate nature of BPDCN and its shared characteristics with pDCs. These include the absence of T cell receptor and immunoglobulin heavy chain gene rearrangements, as well as the production of IFN-alfa (IFN-α) and Th2 polarization following Interleukin-3 (IL-3) induction [21,22,23,24].
In conclusion, successfully addressing the diagnostic challenges posed by BPDCN requires a deep understanding of its immunophenotypic complexities, the strategic use of advanced diagnostic methods like flow cytometry, and staying informed about evolving insights into its cellular origins and molecular characteristics.
1.4. Immunophenotype of BPDCN
The diagnosis of BPDCN hinges on discerning specific immunophenotypic features within neoplastic cells [25]. Typically, BPDCN cells coexpress CD4 and CD56, in addition to CD34, CD36, and pDC-associated antigens like CD123 and CD303. Rare instances may involve an aberrant expression of CD5, CD7, CD33, TdT, and CD79a. The presence of detrimental lineage-specific antigens (Lin-) has also been observed [20,24,26].
A recent study has categorized BPDCN into three maturation-associated subgroups based on the expression of CD34 and CD117 in pDCs. In stage 1, CD34 is expressed in some immature blasts; in stage 2, blast cells partially express CD117, while CD34 is negative; in stage 3, both CD34 and CD117 are negative in blast cells. These distinctive maturation profiles contribute to the diverse clinical presentations and laboratory characteristics of BPDCN.
While the origin and development of pDC malignancies remain debated, contemporary evidence leans towards considering BPDCN cells the malignant counterparts of precursor pDCs [3,7].
1.5. Molecular and Genetic Analyses
Molecular and genetic analyses play a crucial role in the detailed characterization of BPDCN by offering valuable diagnostic and prognostic insights. Cytogenetic techniques, such as karyotyping and fluorescence in situ hybridization (FISH) have been instrumental in identifying frequent chromosomal anomalies associated with BPDCN. Notable abnormalities include deletions or rearrangements of chromosomes 5q, 12p, and 13q, and mutations in the TP53 gene. Further refinement in detection methods comes from next-generation sequencing (NGS), which sheds light on specific genetic mutations. Alterations in genes like Tet methyl cytosine dioxygenase (TET2), ASXL transcriptional regulator 1 (ASXL1), and DNA methyltransferase 3 alpha (DNMT3A) are commonly reported in BPDCN [24,25,26,27,28]. Moreover, chromosomal changes commonly linked to BPDCN include anomalies in regions 5q, 6q, monosomy 9, 12p, 13q, and 15q [18,26,27,28]. In particular, some of these chromosomal deletions prominently affect key genes involved in cell cycle regulation (CDKN2A/CDKN2B and CDKN1B), tumor suppression (RB1, TP53, and PTEN), transcription factors (IKZF1 and ETV6), and other relevant genes (NR3C1 and LATS2) [28].
2. Treatment of BPDCN
2.1. Treatment Strategies for BPDCN
The recognition of BPDCN as a distinct disease entity is a recent development, and due to its rarity, the establishment of standardized treatment protocols is yet to be achieved. Traditionally, conventional chemotherapy has been the primary method of treating BPDCN.
2.2. Conventional Chemotherapy
Chemotherapy regimens originally designed for treating acute leukemia have shown varying degrees of success in inducing sustained clinical responses in BPDCN patients. Regimens that combine anthracycline-based induction therapies with high-dose cytarabine have produced encouraging remission rates. In clinical settings, programs such as HyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) have been employed [2,29]. The determination of the most effective treatment plan, including the regimen and length of therapy, remains contentious, largely due to the scarcity of comprehensive clinical trials and variability in patient profiles.
2.3. Stem Cell Transplantation
In selected BPDCN patients, allogeneic stem cell transplantation (allo-SCT) offers a potentially curative approach and is considered the gold-standard consolidation therapy in patients who attain remission and are fit for the procedure. This, however, often excludes the majority of patients over the age of 65 or those with compromised clinical conditions and comorbidities [5,30,31]. Evidence indicates that conducting allo-SCT during the first complete remission (CR1) or after securing a durable remission through induction chemotherapy can enhance OS rates and decrease relapse risks [4,29]. Nevertheless, refining patient selection criteria, deciding on the optimal timing for transplantation, and determining effective conditioning regimens are all active fields of investigation.
3. Anti-CD123 Chimeric Antigen Receptors T Cell in BPDCN
Autologous T cells engineered to express CAR represent a promising avenue for the treatment of diverse tumors, eliciting a robust T cell immune response that specifically targets and eliminates cancerous cells [60,61,62]. Notably, CAR T cell immunotherapy targeting the pan-B-cell antigen CD19 has demonstrated significant success, particularly in achieving high remission rates among patients with ALL and non-Hodgkin lymphoma (NHL), leading to accelerated FDA approvals in 2017 [63,64]. In the quest for effective T cell-based immunotherapy, CD123 emerges as an attractive target. Preclinical studies have shown that anti-CD123 CAR-T therapy holds substantial promise in the treatment of BPDCN [65]. To mitigate potential adverse effects, such as cytokine release syndrome (CRS) resulting from a robust immune response, these trials have incorporated safety switches in the form of various CAR designs [66,67].
Specifically, T cells from BPDCN patients transduced with CD28/4-1BB CD123 CAR have demonstrated efficacy in vitro, successfully eliminating autologous BPDCN blasts and reducing the BPDCN blast burden in vivo, without causing significant on-target/off-tumor toxicity effects [68]. A recent development in the field is the emergence of TCRαβ-negative allogeneic CAR T cells, often referred to as “universal” CAR T cells (Figure 2). Notably, UCAR T 123, composed of allogeneic T cells from healthy donors expressing anti-CD123 CAR and edited using transcription activator-like effector nuclease (TALEN), presents a promising candidate for treating relapsed/refractory AML and BPDCN (Figure 2) [67].
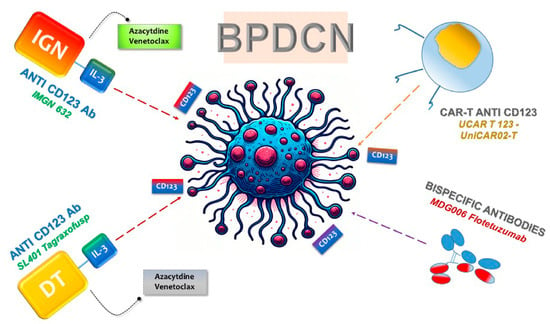
Figure 2. Targeting of CD123 in BPDCN Therapies. The figure illustrates the significant up regulation of CD123 on neoplastic cells, leading to the development of targeted therapeutic strategies. Monoclonal antibodies, BsAbs, and cellular therapies, including chimeric antigen receptor (CAR) T cells, have advanced to early-phase clinical trials for BPDCN. These therapies leverage the specificity for CD123 to selectively target and treat tumor cells.
UCART123 cell therapy, as a salvaging method for BPDCN patients unable to harvest normal T cells for CAR T generation, has demonstrated success in xenograft mouse models with primary patient-derived BPDCN. However, challenges may arise due to the potential loss of the CD123 antigen, as detected in some BPDCN cases. In summary, these results provide a preclinical proof-of-principle that allogeneic UCART123 cells have potent anti-BPDCN activity [67].
Clinical trials, such as NCT03203369 and NCT04109482, are actively assessing the efficacy of anti-CD123 CAR T cells for BPDCN treatment, although the submission of results is still pending, according to ClinicalTrials.gov.
In addition to UCAR T 123, several other CAR-T products are currently under investigation in clinical trials. The noteworthy ones among them are MB-102 (NCT04109482 and NCT02159495) and UniCAR02-T (NCT04230265), both targeted for relapsed or refractory CD123 hematologic neoplasms and BPDCN patients [65,69,70] (Figure 2). Results from two-phase clinical studies (NCT04109482 and NCT02159495) using MB-102 have shown initial responses in four out of seven patients, including one with BPDCN. Meanwhile, a phase I study (NCT04230265) employing UniCAR02-T in patients with relapsed AML, ALL, and BPDCN is currently recruiting, and results are awaited. Notably, UniCAR02-T was built-in, combining UniCAR T cells with the specific targeting of a CD123 recombinant antibody derivative molecule (TM123), making it active against its target only in the presence of TM123 [69].
In conclusion, these developments underscore the significant potential of CAR-T therapies, particularly those targeting CD123, in the treatment of hematologic neoplasms, although ongoing clinical trials will provide critical insights into their real-world effectiveness.
This entry is adapted from the peer-reviewed paper 10.3390/ijms25031454
This entry is offline, you can click here to edit this entry!
