Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Microbiology
The genus Bacillus represents a diverse group of Gram-positive, endospore-forming bacterial species with the well-deserved fame of being potent, versatile, and one of the most promising industrial microorganisms yet discovered. They have an average genome size between 3.4 and 6.0 Mbp. Genetically modified B. subtilis and, to a lesser extent, B. licheniformis, B. amyloliquefaciens, and B. megaterium have been used for the heterologous expression of numerous proteins (enzymes, vaccine components, growth factors), platform chemicals, and other organic compounds of industrial importance.
- Bacillus
- enzymes
- growth factors
- vitamins
- amino acids
- antimicrobial peptide
1. Introduction
The genus Bacillus represents a diverse group of Gram-positive, endospore-forming bacterial species with the well-deserved fame of being potent, versatile, and one of the most promising industrial microorganisms yet discovered. They have an average genome size between 3.4 and 6.0 Mbp [1] and a low GC% content ranging from ~35% in B. thuringiensis to 43.5–46.4% in B. subtilis, B. licheniformis, and B. velezensis [2,3,4,5]. The distinct advantages of Bacillus spp. used as microbial cell factories include a short fermentation cycle (around 48 h), ease of cultivation, and robust growth; non-pathogenic Generally Regarded as Safe (GRAS) status; the ability to secrete recombinant proteins in the medium; and the lack of external and endotoxins [1,6]. In recent decades, genetically modified B. subtilis and other Bacillus spp., notably B. licheniformis, B. amyloliquefaciens, and B. megaterium, have been used prodigiously for the heterologous production of anything from pharmaceutical proteins (antibody fragments, interferons and interleukins, growth factors, hormone precursors, and antimicrobial peptides) to industrial and food-grade enzymes. Large-scale genetic engineering has made possible the redirection of whole metabolic pathways toward valuable non-protein products such as organic acids, alcohols, and vitamins. Compared to Escherichia coli, its chief rival among recombinant bacteria [7], Bacillus spp. used to have some limitations in the past associated with the relatively smaller number of suitable regulatory regions for gene expression, peculiarities in the secretion of recombinant proteins, and the need for the selection of domesticated host strains [8]. This situation has been rapidly changed with the development of increasingly diverse and versatile vectors, novel genome editing tools such as the CRISPR/Cas9 system, and the construction of convenient strains deficient in multiple proteases.
An enormous variety of enzymes, growth factors, vitamins, peptides, amino acids, and low-MW compounds have been expressed in recombinant Bacillus spp. (Figure 1), often on a scale with industrial promise. Through the optimization of expression systems and developments in the field of bioengineering and the use of recombinant Bacillus strains, the highest values of industrially important target products have been achieved (Table 1).
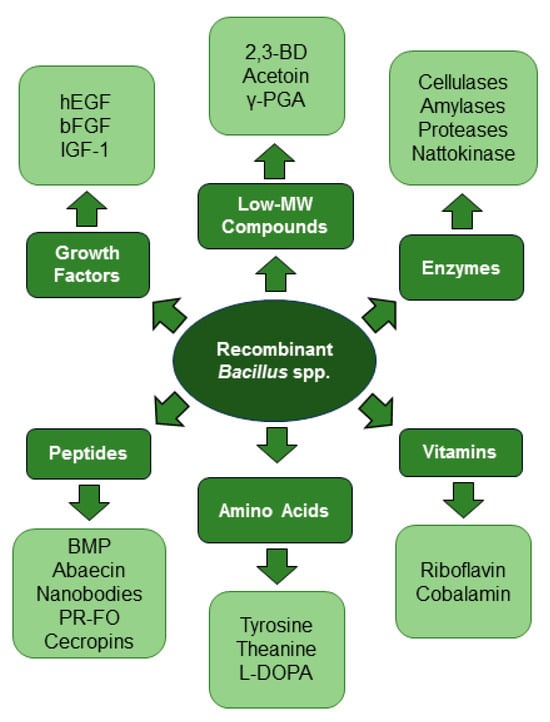
Figure 1. Biotechnological versatility of Bacillus spp.
2. Enzymes
Enzymes with applications in the food industry have predictably been in the spotlight. Genetic improvement of bacilli for the production of α-amylase leads to a gradual increase in the yields obtained (Table 1). The goal of enhanced extracellular expression is achieved through signal peptide optimization and chaperone overexpression [55], the prevention of extracellular degradation by improving the folding environment [56], as well as by complex balancing of the entire secretion process [57]. Thus, the recombinant strain B. subtilis WHS9GSAB produced 35,779.5 U/mL α-amylase for 93 h, reaching a productivity of 384.7 U/mL/h [58].
Table 1. Application of recombinant Bacillus spp. with the highest production of industrially important compounds.
| Strain | Vector | Compound | Genetic Source | Yield | Reference |
|---|---|---|---|---|---|
| B. subtilis WHS11YSA | pHYYamySA | α-amylase | B. stearothermophilus | 9201.1 U/mL | [55] |
| Brevibacillus choshinensis (B. brevis) BCPPSQ | pNCamyS-prsQ | α-amylase | B. stearothermophilus | 17,925.6 U/mL | [56] |
| B. subtilis WHS9GSAB | pHYGamySAsecYEG | α-amylase | B. stearothermophilus | 35,779.5 U/mL | [57] |
| Br. choshinensis (B. brevis) | pNCMO2 | β-amylase | B. aryabhattai CCTCC M2017320 | 5371.8 U/mL | [58] |
| B. subtilis WS9PUL | pHYcas9 | pullulanase | B. deramificans | 5951.8 U/mL | [59] |
| B. subtilis WB600 | pMA5 | lipase A | B. subtilis | 1164.9 U/mL | [60] |
| B. subtilis DB10 | pSKE194 | xylanase | B. subtilis | 1296 U/mg | [61] |
| B. licheniformis MW3 | pKVM1 | 2,3-butanediol | B. licheniformis | 123.7 g/L | [62] |
| B. amyloliquefaciens B 10-127 | pMA5 | 2,3-butanediol | B. amyloliquefaciens | 132.9 g/L | [63] |
| B. subtilis 168 | pMA5 | acetoin | B. subtilis | 91.8 g/L | [64] |
| B. subtilis KH2 | pKVM1 pMA5 |
poly-γ-glutamic acid | B. subtilis, B. licheniformis |
23.28 g/L | [65] |
| B. subtilis G600 | T7-BOOST * | GABA † | B. subtilis | 109.8 g/L | [66] |
* T7-BOOST, T7-Based Optimized Output Strategy for Transcription, a system based on the inducible promoters Phy-spank and PxylA; † GABA, γ-aminobutyric acid.
B. subtilis host strain 1A751 was engineered to express cellobiose 2-epimerase (CEase) from Thermoanaerobacterium saccharolyticum JW/SL-YS485. CEase is a curious enzyme able to convert lactose to epilactose (4-O-β-d-galactopyranosyl-d-mannose), an epimer of lactose difficult to obtain with purely chemical synthesis. While lactose is one of the main by-products in the cheese industry, epilactose reportedly has prebiotic properties [67]. Food-grade maltogenic amylase (AmyM) was produced in the B. subtilis expression system based on the dal gene auxotrophic selection marker. The dal gene was deleted via the knockout plasmid pHYcas9dD, enabling selection with D-alanine instead of an antibiotic. The amylase activity reached 1364 U/mL on shake-flask cultivation and was scaled up to 2388 U/mL in a 3 L fermenter. The addition of maltogenic amylase in the process of breadmaking may extend the shelf life of bread [68].
Proteolytic enzymes used as detergents in the washing industry have received much attention. A metalloprotease from Planococcus sp. 11815 was expressed in Bacillus licheniformis 2709 and showed almost four times higher enzymatic activity (1186 vs. 291 U/mL) compared to the original bacteria [69]. Thermostable serine protease TTHA0724 from Thermus thermophilus HB8 was expressed in B. subtilis RIK1285 16.7 times more effectively than in E. coli. The enzyme showed promise as a detergent additive, being able to eliminate mites and completely clean protein stains at 60 °C. TTHA0724 may be of some use even in the food industry because of its ability to produce active soybean peptides with antioxidant properties at 75 °C [70].
The utilization of plant biomass, the most abundant and renewable energy source on the planet, rich in cellulose and other polysaccharides normally hard to degrade, has received a great deal of scientific attention. Although in this case Bacillus spp. are usually used as a genetic pool for the expression of target enzymes in E. coli, there are some interesting results with genetically modified Bacillus spp. as well. Recombinant B. subtilis DB104, harboring a pSKE194 plasmid with a gene for endoxylanase from B. subtilis AQ1, demonstrated a higher ability to degrade xylan than the non-recombinant strain. The xylanase activity was further increased twice in a 4.5 L fermenter with an inexpensive medium from agricultural waste, finally reaching 602 U/mL after 48 h. Xylan, the second most abundant plant polysaccharide after cellulose, is the source of valuable derivatives such as xylooligosaccharides (XOS), potentially useful as prebiotics in functional foods [61].
Recombinant cellulolytic Bacillus spp. have proven more challenging to produce and maintain on an industrial scale [71]. On a smaller scale, the genes cel8A and cel48S, encoding crucial components of the cellulosome in Acetivibrio thermocellus, were introduced into B. licheniformis 24 and B. velezensis 5RB, respectively, and produced 7-fold higher cellulose activity after 72 h of fermentation in both cases [49].
Heterologous enzymes can be expressed in Bacillus as a strategy designed for the biosynthesis or biodegradation of any compound of interest. Production of trehalose, a non-reducing disaccharide widely used in foodstuffs, cosmetics, pharmaceuticals, and agricultural products, was achieved via one-step fermentation in Bacillus subtilis SCK6, especially engineered for the purpose by introducing into it maltooligosyltrehalose synthase (MTSase) and maltooligosyltrehalose trehalohydrolase (MTHase) from Arthrobacter ramosus S34. Pullulanase PulA was co-expressed to produce the recombinant strain B. subtilis PSH02, which achieved 80% trehalose conversion at 100 g/L maltodextrin [72]. Recombinant B. subtilis DB100, carrying a pUB110-derived plasmid with an alkaline serine protease with keratinolytic activity, has been used for solid-state fermentation (SSF) of feather waste, which is 91% β-keratin and thus a valuable by-product [73].
3. Growth Factors, Vitamins, and Amino Acids
Growth factors have a long and rich expression history in Bacillus. One recent study achieved 104 mg/L human Epidermal Growth Factor (hEGF) within 24 h in B. subtilis DB-104 under the control of an optimized promoter, Psdp-4 [74]. This is a spectacular amount compared to the now historical value of 7 mg/L hEGF obtained a quarter of a century ago [75], not to mention the less than 1.2 g/L previously achieved by B. brevis HDP31, which, moreover, took 6 days to accumulate [76]. However, it must be noted that the quantification of Jun et al. [69] was conducted with software processing of protein bands on SDS-PAGE and should be confirmed with a more reliable quantitative method. The insulin-like growth factor 1 (IGF-1), a small peptide of 70 amino acids vital for gastrointestinal health, and the basic fibroblast growth factor (bFGF), a key player in wound healing, have also been expressed in recombinant Bacillus. B. subtilis WB800N, pHT43 vector, and the novel fusion tag DAMP4 were used to obtain 17 mg/L IGF-1/DAMP4 fusion protein, but only 2.5 mg/L tag-free recombinant IGF-1—a tiny but not unusual amount for that growth factor [77]. B. subtilis 1A751 was engineered to produce bFGF fused to the cellulose-binding domain (CellBD) of the endoglucanase gene cenA from Cellulomonas fimi and ssp DnaB, one of the so-called “inteins” (also known as “protein introns”) found in some bacteria and widely employed for expression and purification of recombinant proteins. The result was the auto-processing of the CellBD-DnaB-bFGF fusion construct and the relatively high yield of 84 mg/L of biologically active bFGF [78].
Vitamins have also been successfully expressed in Bacillus, but not necessarily in industrial amounts. Cobalamin (Vitamin B12) production by B. megaterium ATCC10778 was achieved as far back as 1986, though in rather small amounts: about 26 μg/L [79]. Much more recently, B. megatherium DSM509 was subjected to overexpression of the genes responsible for cobalamin biosynthesis, particularly the operons hemAXCDBL (6.2 kb) and cobl (10.5 kb), under a strong promoter induced by xylose (PxylA) and via chromosomal integration with the vector pHBintE. Despite the 8- to 20-fold increase in the intracellular concentration of cobalamin in transformants compared to the wild type, the final vitamin concentrations remained very low, 1–1.5 μg/L [80]. Riboflavin (Vitamin B2) has been obtained from B. subtilis RX10 via overexpression of ykgB, encoding 6-phosphogluconate-1,5-lactonase, the enzyme catalyzing the second step of the Pentose Phosphate Pathway. This metabolic readjustment assured increased levels of ribose-5-phosphate, a major substrate, and ultimately 7 g/L riboflavin [81]. An even more impressive result was achieved with B. subtilis RF1, in which, among other things, the vgb gene encodes hemoglobin from Vitreoscilla sp. As a result of the increased oxygen utilization, the production of riboflavin was 45.51% higher than the parent strain and reached 10.71 g/L in a 5 L bioreactor [82]. Riboflavin production by Bacillus has been the subject of other remarkable feats of metabolic engineering, as discussed in more detail later (Section 5.2).
Some work has been carried out on amino acids, including some of their valuable derivatives. The promising drug for treating Parkinson’s disease, l-DOPA (3-Hydroxy-l-tyrosine), was produced in substantial amounts by an engineered strain, Bacillus licheniformis, in which, among other things, a tyrosine hydroxylase from Streptosporangium roseum DSM 43021 was introduced. The highest yield reached was 167 mg/L in shake flasks (2.41 times higher than the parent strain) and 1290 mg/L in a 15 L bioreactor [83].
4. Antimicrobial and Immunization Peptides
Antimicrobial peptides (AMPs) have received much attention in recent years as an alternative to antibiotics. Abaecin, an antimicrobial peptide from Apis mellifera that acts as an enhancer of the pore-forming effect of antimicrobial peptides, was expressed and purified from the supernatant of B. subtilis. The recombinant abaecin did not inhibit the growth of E. coli K88, but it did enhance the effect of sublethal doses of cecropin A and hymenoptaecin, both bactericidal proteins isolated from the venom of Apis mellifera [84]. Porcine β-defensin-2 (pBD-2) and cecropin P1 (CP1) were expressed as a fusion antimicrobial peptide in B. subtilis 168 via the pMK4 vector. pBD2-CP1 was digested by enterokinase, and the separate peptides were tested against various pathogens (E. coli, Salmonella typhimurium, Haemophilus parasuis, and Staphylococcus aureus), revealing potent antimicrobial activities. The recombinant B. subtilis strain was shown to promote the health of piglets when added to their feed [85]. A novel antimicrobial peptide derived from the large yellow croaker (Larimichthys crocea) was identified via the expression system of B. subtilis SCK6. The new peptide was given the name Lc1687, was found to consist of 51 amino acids, and showed strong activity against various Gram (−) and Gram (+) pathogens, including St. aureus and Vibrio vulnificus [86].
Recombinant strains of B. subtilis have entered even the field of immunology as vital components of vaccines. B. subtilis, capable of secreting the capsid protein (Cap) of porcine circovirus type 2 (PCV2), one of the most serious pathogens in pigs worldwide, was found to improve the immune response in mice. The authors used the capsid protein from PCV2d, the type currently prevalent in Chinese pigs, and observed it in the supernatant of recombinant bacteria virus-like particles (VLP) of PCV2d Cap protein [87]. Oral immunization of chickens with B. subtilis expressing the multi-epitope protein OmpC-FliC-SopF-SseB-IL-18 was shown to stimulate their immune response towards Salmonella Enteritidis, a major threat for poultry and the cause of massive economic losses [88].
5. Low-MW Compounds
Platform chemicals like 2,3-butanediol (2,3-BD) and acetoin, previously derived from oil, can now be obtained in a more environment-friendly way. A number of microorganisms, including many Bacillus spp., have demonstrated strong producing abilities, in a few exceptional cases reaching about 15%, after the proper genetic modification [89].
In the case of 2,3-BD, microbial production can be tailored to specific stereoisomers; three of them exist for 2,3-BD, a meso compound, and two enantiomers, D(−) and L(+). Meso-2,3-butanediol production has been substantially achieved with B. licheniformis WX-02, 98 g/L [90], and with B. subtilis BSF9, 103.7 g/L [91]. The latter study abolished the production of D(−)-2,3-BD by deleting the gene for the respective butanediol dehydrogenase (BDH). Two stereospecific BDHs were also identified in B. licheniformis MW3. Their deletion yielded two different strains capable of producing 90.1 g/L of the meso compound after 32 h of fermentation and 123.7 g/L of the D(−) enantiomer after 42 h of fermentation [62]. The most spectacular 2,3-BD titers so far have been achieved with B. amyloliquefaciens B10-127, 102.3 g/L [92] and 132.9 g/L [63], both times by sophisticated manipulation of the NADH/NAD+ system and selective knock-out of relevant genes. In these cases, however, the exact isomer is not specified.
From an industrial point of view, the price of the substrate is no less important than the final amount of the product. Broadening the substrate spectrum into cheaper regions is a perennial challenge in industrial microbiology, including 2,3-BD production. B. licheniformis 24, a native superproducer of 2,3-BD, was designed to utilize inulin, a cheap and renewable polysaccharide from plant biomass; for this purpose, an inulinase (fructan-β-fructosidase) from Lacticaseibacillus paracasei DSM 23505 was cloned into the pBE-S shuttle vector and heterologously expressed. While the overall production of 2,3-BD remained low (18.5 g/L after 7 days of fermentation with 200 g/L chicory flour), the recombinant B. licheniformis 24 showed more than 50% higher titers of 2,3-BD and acetoin combined than the wild type after 6 days of fermentation. Moreover, while the wild type’s 2,3-BD titer declined sharply in the next three days, that of the recombinant was correspondingly increased. This curious effect produced a 7-fold gap between the strains after 9 days of fermentation [93]. Acetoin, which is not only a platform chemical but a popular flavor, has been synthesized by various recombinant strains of B. subtilis, such as BS-ppb11, which achieved 82.2 g/L [94]. A metabolically engineered version of B. subtilis 168 reached 91.8 g/L acetoin and 2.3 g/L/h productivity, mostly due to a decreased NADH/NAD+ ratio (2.2-fold) [64]. Somewhat lower but still impressive titers have been obtained with B. licheniformis strains: 64 g/L with MW3 [95] and 79 g/L with WX0279 [96]. The main genetic modification in both cases was the deletion of budC (2,3-butanediol dehydrogenase) and gdh (glycerol dehydrogenase).
This entry is adapted from the peer-reviewed paper 10.3390/fermentation10010050
This entry is offline, you can click here to edit this entry!
