The neglected tropical disease schistosomiasis is a worm infection that is caused by parasitic blood flukes. The disease is found in sub-Saharan Africa, the Middle East, Southeast Asia, and the New World. Worldwide, 240 million people are infected, and 700 million people are at risk. Schistosomiasis is a debilitating, chronic disease, and the mortality is estimated at 200,000 deaths per year. Schistosomiasis control relies on the drug praziquantel, but it does not prevent reinfection after treatment. The development of new vaccines, drugs, and diagnostic methods and the investigation of the biological basis of infectivity are, therefore, of critical importance. The development of transgenesis systems, as have been used for other pathogens, has been hampered by the complexity of the parasite and its life cycle.
- schistosomiasis
- transfection
- functional genomics
- mobile genetic elements
- RNA interference
- RNAi
- genome editing
- CRISPR
- genomic safe harbour site
1. Introduction
2. Biolistics
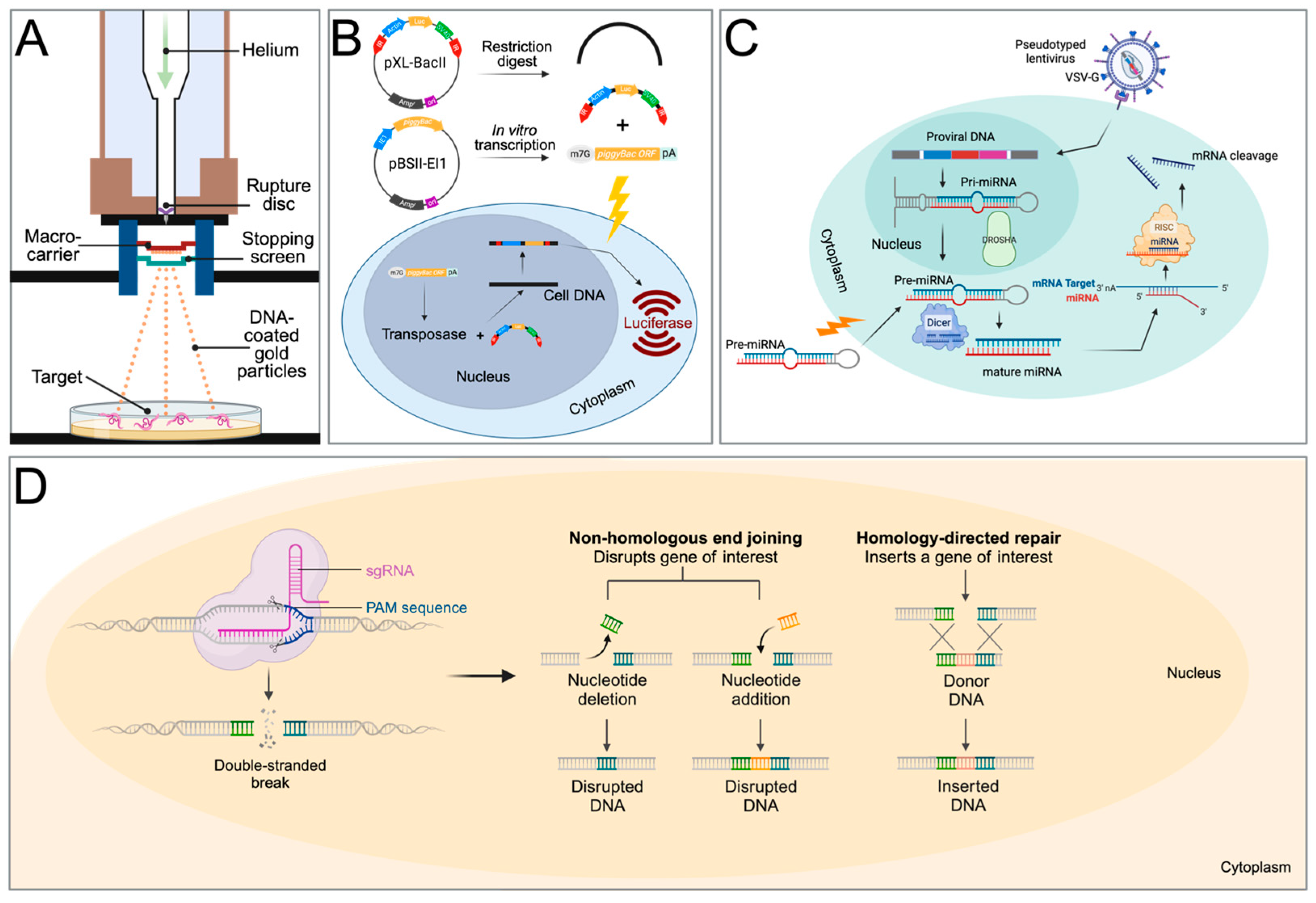
3. Transposable Elements
4. RNA Interference
RNAi In Vivo
5. Highlights of Findings from RNAi Studies
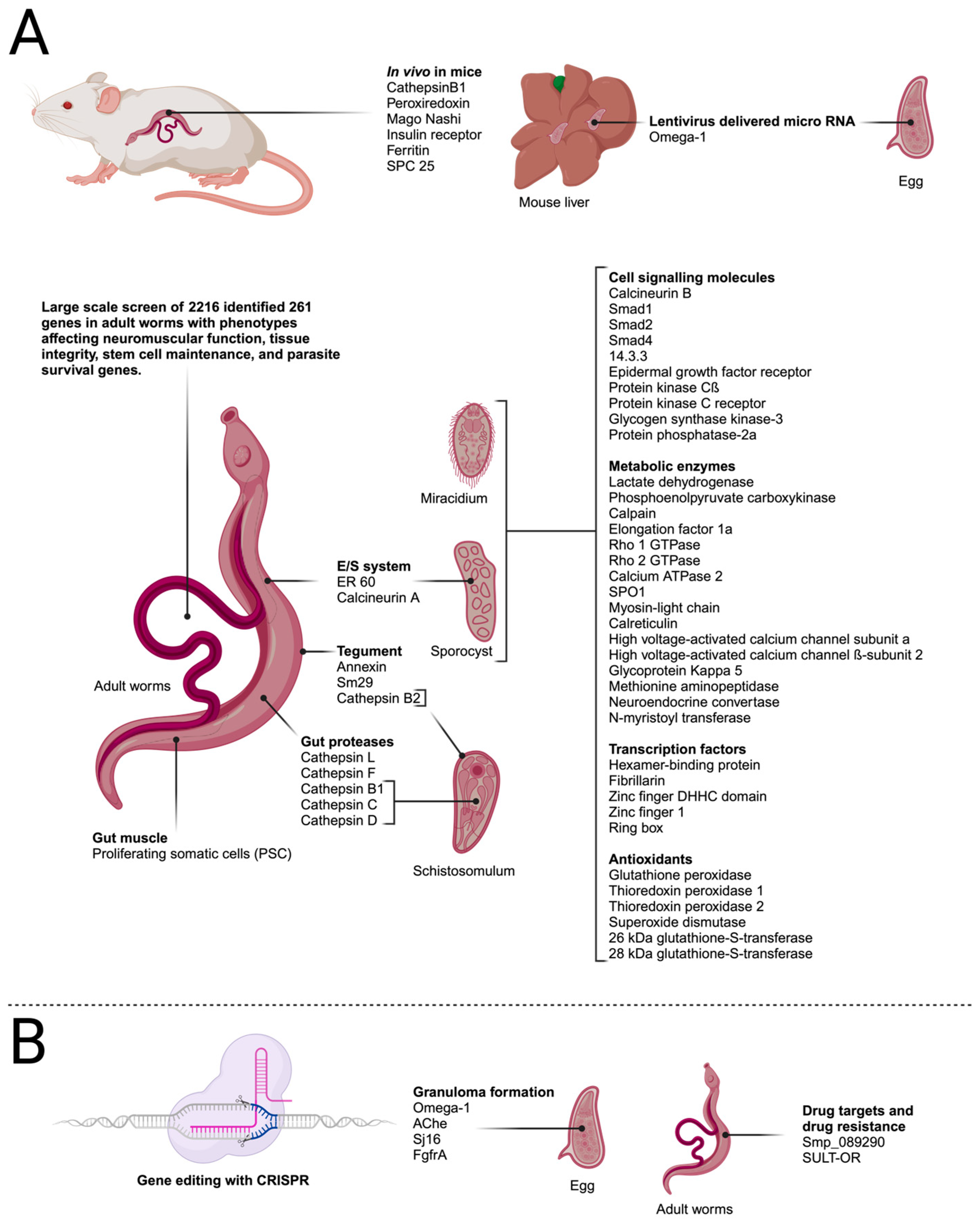
This entry is adapted from the peer-reviewed paper 10.3390/biology13010048
References
- Verjee, M.A. Schistosomiasis: Still a Cause of Significant Morbidity and Mortality. Res. Rep. Trop. Med. 2019, 10, 153–163.
- WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020.
- Schwartz, C.; Fallon, P.G. Schistosoma “Eggs-Iting” the Host: Granuloma Formation and Egg Excretion. Front. Immunol. 2018, 9, 2492.
- Masamba, P.; Kappo, A.P. Immunological and Biochemical Interplay between Cytokines, Oxidative Stress and Schistosomiasis. Int. J. Mol. Sci. 2021, 22, 7216.
- Tokplonou, L.; Nouatin, O.; Sonon, P.; M’po, G.; Glitho, S.; Agniwo, P.; Gonzalez-Ortiz, D.; Tchégninougbo, T.; Ayitchédji, A.; Favier, B.; et al. Schistosoma haematobium Infection Modulates Plasmodium falciparum Parasite Density and Antimalarial Antibody Responses. Parasite Immunol. 2020, 42, e12702.
- Ahmed, A.F.M.; El-Sayad, M.H.; Ali, H.S.; El-Taweel, H.A. Impact of Coinfection with Schistosoma mansoni on the Antibody Response to Helicobacter pylori. Acta Parasitol. 2021, 66, 857–862.
- Muir, R.; Metcalf, T.; Fourati, S.; Bartsch, Y.; Kyosiimire-Lugemwa, J.; Canderan, G.; Alter, G.; Muyanja, E.; Okech, B.; Namatovu, T.; et al. Schistosoma mansoni Infection Alters the Host Pre-Vaccination Environment Resulting in Blunted Hepatitis B Vaccination Immune Responses. PLoS Negl. Trop. Dis. 2023, 17, e0011089.
- Floudas, A.; Aviello, G.; Schwartz, C.; Jeffery, I.B.; O’Toole, P.W.; Fallon, P.G. Schistosoma mansoni Worm Infection Regulates the Intestinal Microbiota and Susceptibility to Colitis. Infect. Immun. 2019, 87, e00275-19.
- Li, Z.; Wang, X.; Zhang, W.; Yang, W.; Xu, B.; Hu, W. Excretory/Secretory Products from Schistosoma japonicum Eggs Alleviate Ovalbumin-Induced Allergic Airway Inflammation. PLoS Negl. Trop. Dis. 2023, 17, e0011625.
- Davis, R.E.; Parra, A.; LoVerde, P.T.; Ribeiro, E.; Glorioso, G.; Hodgson, S. Transient Expression of DNA and RNA in Parasitic Helminths by Using Particle Bombardment. Proc. Natl. Acad. Sci. USA 1999, 96, 8687–8692.
- Wippersteg, V.; Kapp, K.; Kunz, W.; Jackstadt, W.P.; Zahner, H.; Grevelding, C.G. HSP70-Controlled GFP Expression in Transiently Transformed Schistosomes. Mol. Biochem. Parasitol. 2002, 120, 141–150.
- Wippersteg, V.; Kapp, K.; Kunz, W.; Grevelding, C.G. Characterisation of the Cysteine Protease ER60 in Transgenic Schistosoma mansoni Larvae. Int. J. Parasitol. 2002, 32, 1219–1224.
- Wippersteg, V.; Ribeiro, F.; Liedtke, S.; Kusel, J.R.; Grevelding, C.G. The Uptake of Texas Red-BSA in the Excretory System of Schistosomes and Its Colocalisation with ER60 Promoter-Induced GFP in Transiently Transformed Adult Males. Int. J. Parasitol. 2003, 33, 1139–1143.
- Wippersteg, V.; Sajid, M.; Walshe, D.; Khiem, D.; Salter, J.P.; McKerrow, J.H.; Grevelding, C.G.; Caffrey, C.R. Biolistic Transformation of Schistosoma mansoni with 5′ Flanking Regions of Two Peptidase Genes Promotes Tissue-Specific Expression. Int. J. Parasitol. 2005, 35, 583–589.
- Dvořák, J.; Beckmann, S.; Lim, K.-C.; Engel, J.C.; Grevelding, C.G.; McKerrow, J.H.; Caffrey, C.R. Biolistic Transformation of Schistosoma mansoni: Studies with Modified Reporter-Gene Constructs Containing Regulatory Regions of Protease Genes. Mol. Biochem. Parasitol. 2010, 170, 37–40.
- Rossi, A.; Wippersteg, V.; Klinkert, M.-Q.; Grevelding, C.G. Cloning of 5′ and 3′ Flanking Regions of the Schistosoma mansoni Calcineurin A Gene and Their Characterization in Transiently Transformed Parasites. Mol. Biochem. Parasitol. 2003, 130, 133–138.
- Heyers, O.; Walduck, A.K.; Brindley, P.J.; Bleiss, W.; Lucius, R.; Dorbic, T.; Wittig, B.; Kalinna, B.H. Schistosoma mansoni Miracidia Transformed by Particle Bombardment Infect Biomphalaria Glabrata Snails and Develop into Transgenic Sporocysts. Exp. Parasitol. 2003, 105, 174–178.
- Beckmann, S.; Wippersteg, V.; El-Bahay, A.; Hirzmann, J.; Oliveira, G.; Grevelding, C.G. Schistosoma mansoni: Germ-Line Transformation Approaches and Actin-Promoter Analysis. Exp. Parasitol. 2007, 117, 292–303.
- WormBase Parasite. Schistosoma mansoni. Available online: https://parasite.wormbase.org/Schistosoma_mansoni_prjea36577/Info/Index (accessed on 7 November 2023).
- WormBase Parasite. Schistosoma japonicum. Available online: https://parasite.wormbase.org/Schistosoma_japonicum_prjna520774/Info/Index (accessed on 7 November 2023).
- Wilson, R.A.; Ashton, P.D.; Braschi, S.; Dillon, G.P.; Berriman, M.; Ivens, A. Oming in on Schistosomes: Prospects and Limitations for Post-Genomics. Trends Parasitol. 2007, 23, 14–20.
- Haas, B.J.; Berriman, M.; Hirai, H.; Cerqueira, G.G.; LoVerde, P.T.; El-Sayed, N.M. Schistosoma mansoni Genome: Closing in on a Final Gene Set. Exp. Parasitol. 2007, 117, 225–228.
- Brindley, P.J.; Laha, T.; McManus, D.P.; Loukas, A. Mobile Genetic Elements Colonizing the Genomes of Metazoan Parasites. Trends Parasitol. 2003, 19, 79–87.
- Wells, J.N.; Feschotte, C. A Field Guide to Eukaryotic Transposable Elements. Annu. Rev. Genet. 2020, 54, 539–561.
- Laha, T.; Loukas, A.; Verity, C.K.; McManus, D.P.; Brindley, P.J. Gulliver, a Long Terminal Repeat Retrotransposon from the Genome of the Oriental Blood Fluke Schistosoma japonicum. Gene 2001, 264, 59–68.
- DeMarco, R.; Kowaltowski, A.T.; Machado, A.A.; Soares, M.B.; Gargioni, C.; Kawano, T.; Rodrigues, V.; Madeira, A.M.B.N.; Wilson, R.A.; Menck, C.F.M.; et al. Saci-1, -2, and -3 and Perere, Four Novel Retrotransposons with High Transcriptional Activities from the Human Parasite Schistosoma mansoni. J. Virol. 2004, 78, 4950.
- Laha, T.; Loukas, A.; Smyth, D.J.; Copeland, C.S.; Brindley, P.J. The Fugitive LTR Retrotransposon from the Genome of the Human Blood Fluke, Schistosoma mansoni. Int. J. Parasitol. 2004, 34, 1365–1375.
- Feschotte, C. Merlin, a New Superfamily of DNA Transposons Identified in Diverse Animal Genomes and Related to Bacterial IS1016 Insertion Sequences. Mol. Biol. Evol. 2004, 21, 1769–1780.
- DeMarco, R.; Venancio, T.M.; Verjovski-Almeida, S. SmTRC1, a Novel Schistosoma mansoni DNA Transposon, Discloses New Families of Animal and Fungi Transposons Belonging to the CACTA Superfamily. BMC Evol. Biol. 2006, 6, 89.
- Copeland, C.S.; Brindley, P.J.; Heyers, O.; Michael, S.F.; Johnston, D.A.; Williams, D.L.; Ivens, A.C.; Kalinna, B.H. Boudicca, a Retrovirus-like Long Terminal Repeat Retrotransposon from the Genome of the Human Blood Fluke Schistosoma mansoni. J. Virol. 2003, 77, 6153–6166.
- Llorens, C.; Muñoz-Pomer, A.; Bernad, L.; Botella, H.; Moya, A. Network Dynamics of Eukaryotic LTR Retroelements beyond Phylogenetic Trees. Biol. Direct 2009, 4, 41.
- Bae, Y.-A.; Moon, S.-Y.; Kong, Y.; Cho, S.-Y.; Rhyu, M.-G. CsRn1, a Novel Active Retrotransposon in a Parasitic Trematode, Clonorchis sinensis, Discloses a New Phylogenetic Clade of Ty3/Gypsy-like LTR Retrotransposons. Mol. Biol. Evol. 2001, 18, 1474–1483.
- Abe, H.; Ohbayashi, F.; Shimada, T.; Sugasaki, T.; Kawai, S.; Mita, K.; Oshiki, T. Molecular Structure of a Novel Gypsy-Ty3-like Retrotransposon (Kabuki) and Nested Retrotransposable Elements on the W Chromosome of the Silkworm Bombyx Mori. Mol. Gen. Genet. MGG 2000, 263, 916–924.
- Plasterk, R.H.A.; Izsvák, Z.; Ivics, Z. Resident Aliens: The Tc1/Mariner Superfamily of Transposable Elements. Trends Genet. 1999, 15, 326–332.
- Mann, V.H.; Morales, M.E.; Kines, K.J.; Brindley, P.J. Transgenesis of Schistosomes: Approaches Employing Mobile Genetic Elements. Parasitology 2008, 135, 141–153.
- Copeland, C.S.; Mann, V.H.; Brindley, P.J. Both Sense and Antisense Strands of the LTR of the Schistosoma mansoni Pao-like Retrotransposon Sinbad Drive Luciferase Expression. Mol. Genet. Genom. 2007, 277, 161–170.
- Morales, M.E.; Mann, V.H.; Kines, K.J.; Gobert, G.N.; Fraser, M.J.; Kalinna, B.H.; Correnti, J.M.; Pearce, E.J.; Brindley, P.J. PiggyBac Transposon Mediated Transgenesis of the Human Blood Fluke, Schistosoma mansoni. Faseb. J. 2007, 21, 3479–3489.
- Handler, A.M.; McCombs, S.D.; Fraser, M.J.; Saul, S.H. The Lepidopteran Transposon Vector, PiggyBac, Mediates Germ-Line Transformation in the Mediterranean Fruit Fly. Proc. Natl. Acad. Sci. USA 1998, 95, 7520–7525.
- Skelly, P.J.; Da’dara, A.; Harn, D.A. Suppression of Cathepsin B Expression in Schistosoma mansoni by RNA Interference. Int. J. Parasitol. 2003, 33, 363–369.
- Krautz-Peterson, G.; Radwanska, M.; Ndegwa, D.; Shoemaker, C.B.; Skelly, P.J. Optimizing Gene Suppression in Schistosomes Using RNA Interference. Mol. Biochem. Parasitol. 2007, 153, 194–202.
- Correnti, J.M.; Brindley, P.J.; Pearce, E.J. Long-Term Suppression of Cathepsin B Levels by RNA Interference Retards Schistosome Growth. Mol. Biochem. Parasitol. 2005, 143, 209–215.
- Tchoubrieva, E.B.; Ong, P.C.; Pike, R.N.; Brindley, P.J.; Kalinna, B.H. Vector-Based RNA Interference of Cathepsin B1 in Schistosoma mansoni. Cell. Mol. Life Sci. 2010, 67, 3739–3748.
- Da’dara, A.A.; Skelly, P.J. Gene Suppression in Schistosomes Using RNAi. Methods Mol. Biol. 2015, 1201, 143–164.
- Pereira, T.C.; Evangelista, C.C.S.; Borges, G.; Zanotti-Magalhães, E.M.; Magalhães, L.A.; Lopes-Cendes, I. Applications of RNA Interference in Schistosomiasis: Gene Function Identification and Development of New Therapies. ISRN Parasitol. 2013, 2013, 247036.
- Kalinna, B.H.; Ross, A.G.; Walduck, A.K. Schistosome Transgenesis: The Long Road to Success. Biology 2024, 13, 48. https://doi.org/10.3390/biology13010048
- Li, J.; Xiang, M.; Zhang, R.; Xu, B.; Hu, W. RNA Interference in Vivo in Schistosoma japonicum: Establishing and Optimization of RNAi Mediated Suppression of Gene Expression by Long DsRNA in the Intra-Mammalian Life Stages of Worms. Biochem. Biophys. Res. Commun. 2018, 503, 1004–1010.
- Zeng, F.; Yi, C.; Zhang, W.; Cheng, S.; Sun, C.; Luo, F.; Feng, Z.; Hu, W. A New Ferritin SjFer0 Affecting the Growth and Development of Schistosoma Japonicum. Parasit. Vectors 2022, 15, 177.
- Yang, W.-B.; Luo, F.; Zhang, W.; Sun, C.-S.; Tan, C.; Zhou, A.; Hu, W. Inhibition of Signal Peptidase Complex Expression Affects the Development and Survival of Schistosoma japonicum. Front. Cell. Infect. Microbiol. 2023, 13, 1136056.
- Collins, J.J., 3rd; Wang, B.; Lambrus, B.G.; Tharp, M.E.; Iyer, H.; Newmark, P.A. Adult Somatic Stem Cells in the Human Parasite Schistosoma mansoni. Nature 2013, 494, 476–479.
- Shaw, M.K.; Erasmus, D.A. Schistosoma mansoni: Structural Damage and Tegumental Repair after In Vivo Treatment with Praziquantel. Parasitology 1987, 94, 243–254.
- Mourão, M.M.; Dinguirard, N.; Franco, G.R.; Yoshino, T.P. Phenotypic Screen of Early-Developing Larvae of the Blood Fluke, Schistosoma Mansoni, Using RNA Interference. PLoS. Negl. Trop. Dis. 2009, 3, e502.
- Stefanić, S.; Dvořák, J.; Horn, M.; Braschi, S.; Sojka, D.; Ruelas, D.S.; Suzuki, B.; Lim, K.-C.; Hopkins, S.D.; McKerrow, J.H.; et al. RNA Interference in Schistosoma mansoni Schistosomula: Selectivity, Sensitivity and Operation for Larger-Scale Screening. PLoS. Negl. Trop. Dis. 2010, 4, e850.
- Wang, J.; Paz, C.; Padalino, G.; Coghlan, A.; Lu, Z.; Gradinaru, I.; Collins, J.N.R.; Berriman, M.; Hoffmann, K.F.; Collins, J.J., 3rd. Large-Scale RNAi Screening Uncovers Therapeutic Targets in the Parasite Schistosoma mansoni. Science 2020, 369, 1649–1653.
