Polycystic ovary syndrome (PCOS) is the most common endocrine-metabolic disease in females of reproductive age, affecting 4–20% of pre-menopausal women worldwide. MicroRNAs (miRNAs) are endogenous, single-stranded, non-coding, regulatory ribonucleic acid molecules found in eukaryotic cells. Abnormal miRNA expression has been associated with several diseases and could possibly explain their underlying pathophysiology. MiRNAs have been extensively studied for their potential diagnostic, prognostic, and therapeutic uses in many diseases, such as type 2 diabetes, obesity, cardiovascular disease, PCOS, and endometriosis.
- polycystic ovary syndrome (PCOS)
- microRNA (miRNAs)
- infertility
- cardiovascular disease
- insulin resistance
1. Introduction to Polycystic Ovary Syndrome
2. Introduction to microRNA
3. The Role of miRNA in the Pathogenesis of Common PCOS Complications
3.1. Infertility
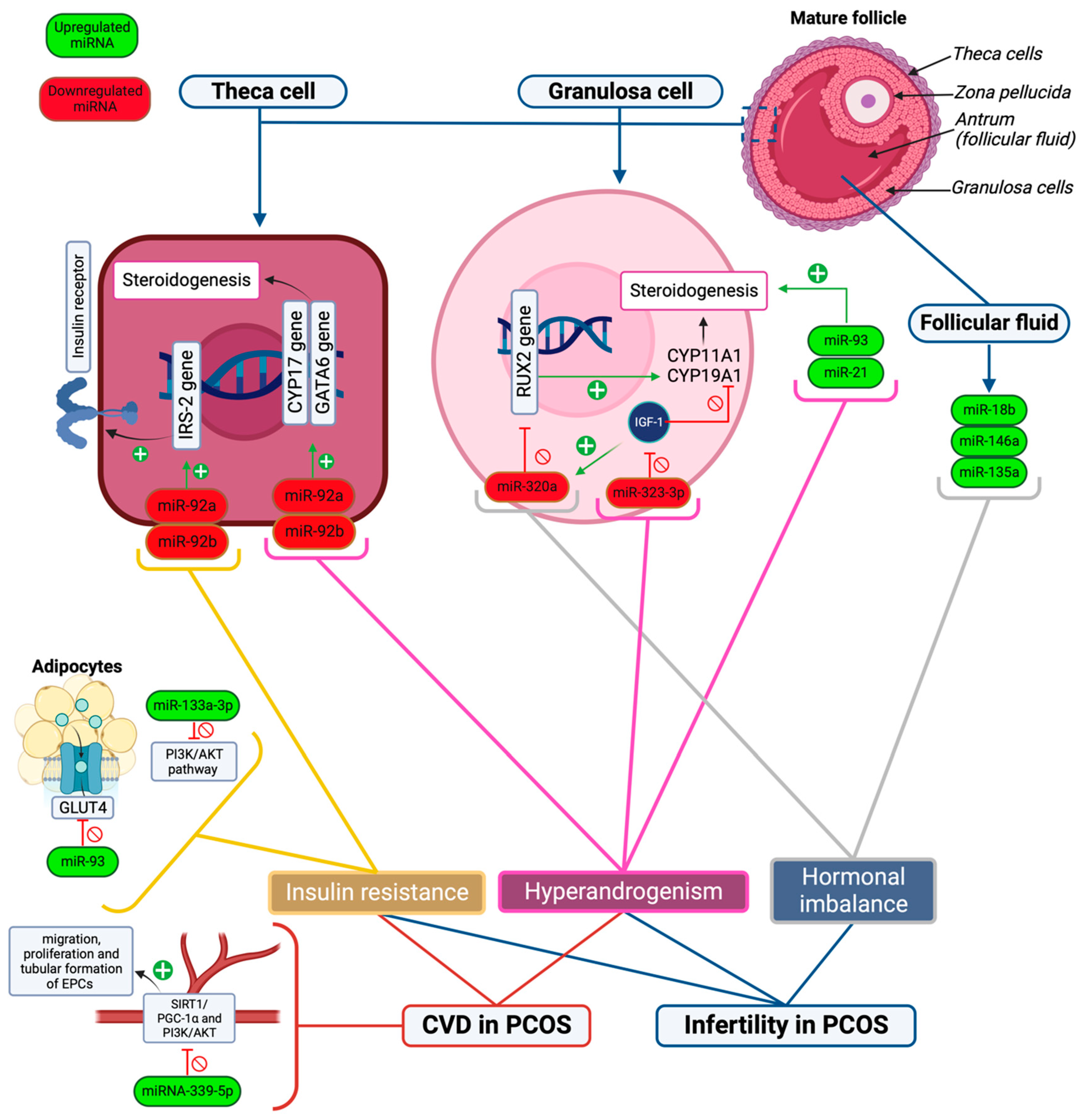
3.2. Insulin Resistance
3.3. Cardiovascular Complications
This entry is adapted from the peer-reviewed paper 10.3390/ijms25020903
References
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336.
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284.
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271.
- Witchel, S.F.; Oberfield, S.E.; Pena, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573.
- Randeva, H.S.; Tan, B.K.; Weickert, M.O.; Lois, K.; Nestler, J.E.; Sattar, N.; Lehnert, H. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr. Rev. 2012, 33, 812–841.
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104.
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520.
- Escobar-Morreale, H.F.; Luque-Ramirez, M.; San Millan, J.L. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr. Rev. 2005, 26, 251–282.
- Charifson, M.A.; Trumble, B.C. Evolutionary origins of polycystic ovary syndrome: An environmental mismatch disorder. Evol. Med. Public Health 2019, 2019, 50–63.
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379.
- Smet, M.E.; McLennan, A. Rotterdam criteria, the end. Australas. J. Ultrasound Med. 2018, 21, 59–60.
- Ranganathan, K.; Sivasankar, V. MicroRNAs—Biology and clinical applications. J. Oral Maxillofac. Pathol. 2014, 18, 229–234.
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402.
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, biogenesis, and their evolving role in animal development and disease. Vet. Pathol. 2014, 51, 759–774.
- Ilie, I.R.; Georgescu, C.E. Polycystic ovary syndrome-epigenetic mechanisms and aberrant microRNA. Adv. Clin. Chem. 2015, 71, 25–45.
- Abdalla, M.; Deshmukh, H.; Atkin, S.L.; Sathyapalan, T. miRNAs as a novel clinical biomarker and therapeutic targets in polycystic ovary syndrome (PCOS): A review. Life Sci. 2020, 259, 118174.
- Flynt, A.S.; Lai, E.C. Biological principles of microRNA-mediated regulation: Shared themes amid diversity. Nat. Rev. Genet. 2008, 9, 831–842.
- Xue, Y.; Lv, J.; Xu, P.; Gu, L.; Cao, J.; Xu, L.; Xue, L.; Li, Q. Identification of microRNAs and genes associated with hyperandrogenism in the follicular fluid of women with polycystic ovary syndrome. J. Cell. Biochem. 2018, 119, 3913–3921.
- Vitale, S.G.; Fulghesu, A.M.; Mikus, M.; Watrowski, R.; D’Alterio, M.N.; Lin, L.T.; Shah, M.; Reyes-Muñoz, E.; Sathyapalan, T.; Angioni, S. The Translational Role of miRNA in Polycystic Ovary Syndrome: From Bench to Bedside—A Systematic Literature Review. Biomedicines 2022, 10, 1816.
- Imbar, T.; Eisenberg, I. Regulatory role of microRNAs in ovarian function. Fertil. Steril. 2014, 101, 1524–1530.
- Hwang, H.; Chang, H.R.; Baek, D. Determinants of Functional MicroRNA Targeting. Mol. Cells 2023, 46, 21–32.
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167.
- Mari-Alexandre, J.; Barcelo-Molina, M.; Belmonte-Lopez, E.; Garcia-Oms, J.; Estelles, A.; Braza-Boils, A.; Gilabert-Estellés, J. Micro-RNA profile and proteins in peritoneal fluid from women with endometriosis: Their relationship with sterility. Fertil. Steril. 2018, 109, 675–684.e2.
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids—The mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477.
- Li, P.; Liu, H.; Li, Y.; Wang, Y.; Zhao, L.; Wang, H. miR-339-5p inhibits lung adenocarcinoma invasion and migration by directly targeting BCL6. Oncol. Lett. 2018, 16, 5785–5790.
- Shan, W.; Li, J.; Bai, Y.; Lu, X. miR-339-5p inhibits migration and invasion in ovarian cancer cell lines by targeting NACC1 and BCL6. Tumor Biol. 2016, 37, 5203–5211.
- Sathyapalan, T.; David, R.; Gooderham, N.J.; Atkin, S.L. Increased expression of circulating miRNA-93 in women with polycystic ovary syndrome may represent a novel, non-invasive biomarker for diagnosis. Sci. Rep. 2015, 5, 16890.
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452.
- Chen, B.; Xu, P.; Wang, J.; Zhang, C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene 2019, 706, 91–96.
- Motahari Rad, H.; Mowla, S.J.; Ramazanali, F.; Rezazadeh Valojerdi, M. Characterization of altered microRNAs related to different phenotypes of polycystic ovarian syndrome (PCOS) in serum, follicular fluid, and cumulus cells. Taiwan. J. Obstet. Gynecol. 2022, 61, 768–779.
- Li, M.; Ruan, X.; Mueck, A.O. Management strategy of infertility in polycystic ovary syndrome. Glob. Health J. 2022, 6, 70–74.
- Zhuang, S.; Jing, C.; Yu, L.; Ji, L.; Liu, W.; Hu, X. The relationship between polycystic ovary syndrome and infertility: A bibliometric analysis. Ann. Transl. Med. 2022, 10, 318.
- Sawant, S.; Bhide, P. Fertility Treatment Options for Women with Polycystic Ovary Syndrome. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119890867.
- Gunning, M.N.; Fauser, B. Are women with polycystic ovary syndrome at increased cardiovascular disease risk later in life? Climacteric 2017, 20, 222–227.
- Xu, Y.; Qiao, J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J. Healthc. Eng. 2022, 2022, 9240569.
- Roth, L.W.; McCallie, B.; Alvero, R.; Schoolcraft, W.B.; Minjarez, D.; Katz-Jaffe, M.G. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2014, 31, 355–362.
- Naji, M.; Aleyasin, A.; Nekoonam, S.; Arefian, E.; Mahdian, R.; Amidi, F. Differential Expression of miR-93 and miR-21 in Granulosa Cells and Follicular Fluid of Polycystic Ovary Syndrome Associating with Different Phenotypes. Sci. Rep. 2017, 7, 14671.
- Wang, T.; Liu, Y.; Lv, M.; Xing, Q.; Zhang, Z.; He, X.; Xu, Y.; Wei, Z.; Cao, Y. miR-323-3p regulates the steroidogenesis and cell apoptosis in polycystic ovary syndrome (PCOS) by targeting IGF-1. Gene 2019, 683, 87–100.
- Zhang, C.L.; Wang, H.; Yan, C.Y.; Gao, X.F.; Ling, X.J. Deregulation of RUNX2 by miR-320a deficiency impairs steroidogenesis in cumulus granulosa cells from polycystic ovary syndrome (PCOS) patients. Biochem. Biophys. Res. Commun. 2017, 482, 1469–1476.
- Lin, L.; Du, T.; Huang, J.; Huang, L.L.; Yang, D.Z. Identification of differentially expressed microRNAs in the ovary of polycystic ovary syndrome with hyperandrogenism and insulin resistance. Chin. Med. J. 2015, 128, 169–174.
- Zhao, H.; Zhang, J.; Cheng, X.; Nie, X.; He, B. Insulin resistance in polycystic ovary syndrome across various tissues: An updated review of pathogenesis, evaluation, and treatment. J. Ovarian Res. 2023, 16, 9.
- Chen, Y.H.; Heneidi, S.; Lee, J.M.; Layman, L.C.; Stepp, D.W.; Gamboa, G.M.; Chen, B.S.; Chazenbalk, G.; Azziz, R. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 2013, 62, 2278–2286.
- Balasubramanyam, M.; Aravind, S.; Gokulakrishnan, K.; Prabu, P.; Sathishkumar, C.; Ranjani, H.; Mohan, V. Impaired miR-146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes. Mol. Cell. Biochem. 2011, 351, 197–205.
- Coleman, C.B.; Lightell, D.J., Jr.; Moss, S.C.; Bates, M.; Parrino, P.E.; Woods, T.C. Elevation of miR-221 and -222 in the internal mammary arteries of diabetic subjects and normalization with metformin. Mol. Cell. Endocrinol. 2013, 374, 125–129.
- Capuani, B.; Pacifici, F.; Della-Morte, D.; Lauro, D. Glucagon Like Peptide 1 and MicroRNA in Metabolic Diseases: Focusing on GLP1 Action on miRNAs. Front. Endocrinol. 2018, 9, 719.
- Radbakhsh, S.; Sathyapalan, T.; Banach, M.; Sahebkar, A. Incretins and microRNAs: Interactions and physiological relevance. Pharmacol. Res. 2020, 153, 104662.
- Angioni, S.; Sanna, S.; Magnini, R.; Melis, G.B.; Fulghesu, A.M. The quantitative insulin sensitivity check index is not able to detect early metabolic alterations in young patients with polycystic ovarian syndrome. Gynecol. Endocrinol. 2011, 27, 468–474.
- Cao, J.; Huo, P.; Cui, K.; Wei, H.; Cao, J.; Wang, J.; Liu, Q.; Lei, X.; Zhang, S. Follicular fluid-derived exosomal miR-143-3p/miR-155-5p regulate follicular dysplasia by modulating glycolysis in granulosa cells in polycystic ovary syndrome. Cell Commun. Signal. 2022, 20, 61.
- Guan, C.; Zahid, S.; Minhas, A.S.; Ouyang, P.; Vaught, A.; Baker, V.L.; Michos, E.D. Polycystic ovary syndrome: A “risk-enhancing” factor for cardiovascular disease. Fertil. Steril. 2022, 117, 924–935.
- Meun, C.; Gunning, M.N.; Louwers, Y.V.; Peters, H.; Roos-Hesselink, J.; Roeters van Lennep, J.; Rueda Ochoa, O.L.; Appelman, Y.; Lambalk, N.; Boersma, E.; et al. The cardiovascular risk profile of middle-aged women with polycystic ovary syndrome. Clin. Endocrinol. 2020, 92, 150–158.
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc. Med. 2020, 30, 399–404.
- Gomez, J.M.D.; VanHise, K.; Stachenfeld, N.; Chan, J.L.; Merz, N.B.; Shufelt, C. Subclinical cardiovascular disease and polycystic ovary syndrome. Fertil. Steril. 2022, 117, 912–923.
- Duica, F.; Danila, C.A.; Boboc, A.E.; Antoniadis, P.; Condrat, C.E.; Onciul, S.; Suciu, N.; Creţoiu, S.M.; Varlas, V.N.; Creţoiu, D. Impact of Increased Oxidative Stress on Cardiovascular Diseases in Women with Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 614679.
- Wronska, A. The Role of microRNA in the Development, Diagnosis, and Treatment of Cardiovascular Disease: Recent Developments. J. Pharmacol. Exp. Ther. 2023, 384, 123–132.
- Zhang, J.; Xu, W.; Li, S.; Zhang, J.; Shang, Y.; Gui, J. The role of miRNA-339-5p in the function of vascular endothelial progenitor cells in patients with PCOS. Reprod. Biomed. Online 2022, 44, 423–433.
- Kao, Y.H.; Chiu, W.C.; Hsu, M.I.; Chen, Y.J. Endothelial progenitor cell dysfunction in polycystic ovary syndrome: Implications for the genesis of cardiovascular diseases. Int. J. Fertil. Steril. 2013, 6, 208–213.
