1. Different Types of Polymersomes
Polymersomes can be endowed with high functional versatility relevant to diagnostic and therapeutic applications owing to their synthetic plasticity, and three large groups may be highlighted. One contains polymersomes labeled with imaging molecules. The second includes polymersomes decorated with targeting ligands for selective delivery. The last class of functionalized polymersomes is based on stimuli-responsive polymersomes, able to transport and release therapeutic molecules in a controlled manner and in response to external (light or temperature) or internal (pH, redox potential, or enzymes) stimuli.
1.1. Copolymer Types
There are many copolymers with different compositions, characteristics, and pathways of synthesis, with a characteristic composition that allows the acquisition of polymersome morphology with desired properties to be used in drug delivery systems and other applications.
The most used hydrophilic copolymer is biocompatible poly(ethylene oxide) (PEO), also known as poly(ethylene glycol) (PEG) [
43]. Adjustable length, density, and configuration of the PEG layer can protect a therapeutic load under physiological conditions and delay clearance from the bloodstream [
28]. However, it has some drawbacks, including the use of toxic and gaseous monomer ethylene oxide in polymer synthesis, risk of peroxidation, possible immune response, and protein adsorption [
44]. A recent investigation of the influence of terminal PEG OH groups on the formation of protein corona and polymersome uptake by diverse immune cell subpopulations in different biological compartments could provide valuable insights for the rational design of polymersomes, especially in the field of immunotherapy [
45]. Alternative hydrophilic polymers have also been explored, such as poly(oxazoline)s, poly(sarcosine), and poly(glycidol), or oligosaccharides [
46], because of their biocompatibility and structural and synthetic versatility [
47]. At the same time, they show enhanced hydration and better antifouling properties [
43]. When PDPA was conjugated with (poly([
N-(2-hydroxypropyl)]metha acrylamide)) (PHPMA) hydrophilic moiety, the resulting PHPMA
35-
b-PDPA
42 block copolymer offered advantages in terms of its protein-repelling properties [
31]. Relative to PEG-
b-PLA, PHPMA
35-
b-PDPA
42 block copolymer showed higher stability with inconsiderable protein binding, while PEG-
b-PLA polymersomes proved to be susceptible to protein adsorption in a protein-dependent way. In this case, the length of the hydrophilic shell and the chemical nature of the outer surface had the highest influence on the protein adsorption [
31]. Although the influence of PEGylation parameters has been well studied in various nanosystems, comprehensive studies of the same kind are rare for other types of hydrophilic polymers. To fill this gap, Najer et al. evaluated the influence of poly(2-methyl-2-oxazoline) length on the antifouling properties of the poly(2-methyl-2-oxazoline)-block-poly(dimethylsiloxane)-block-poly(2-methyl-2-oxazoline) (PMOXA-
b-PDMS-
b-PMOXA)-based polymersomes [
1]. Mixtures of polymers with hydrophilic fractions between 43 (n
21-65-21) and 20 (n
6-65-6) % (the limit values for polymersome formation based on f
hydrophilic of ≈35 ± 10%) were used. Maximizing PMOXA length and amount within the mixture allows for maximization of interfacial water layer thickness and density, optimizing the antifouling behavior. Nonetheless, PMOXA length remains bound to the length of the hydrophobic component in the copolymer in order to ensure the formation of stable polymersomes. Although the formulation with the least protein corona performed the best in in vitro and in vivo models, it was inferior to PEGylated liposomes of similar size, offering possibilities for further optimization of polyoxazolines.
The hydrophobic components of polymersomes have also been studied, and special attention has been paid to biodegradable polymers with low in vivo toxicity. They include poly(lactic acid) (PLA), PCL, and poly(trimethylene carbonate) (PTMC) [
28]. A comparison of poly(propylene oxide) (PPO) and poly(butylene oxide) (PBO) concluded that PBO has improved cytocompatibility, higher hydrophobicity, simpler fabrication, and lower glass transition temperature (−70 °C), which is useful when high membrane fluidity and flexibility are needed [
43]. It makes PBO advantageous in relation to other hydrophobic blocks, such as PPO, PLA, or PCL. PLA and PCL need high temperatures during the self-assembly process since they are semi-crystalline and, therefore, cannot be used with biologically active compounds sensitive to high temperatures. Other hydrophobic moieties can also be used. Cholesterol (Chol) and α-tocopherol are biological, lipophilic, and biocompatible molecules used for the core construction of self-assemblies [
48,
49]. Polystyrene (PS) is a hydrophobic, FDA-approved copolymer that can be used for API-free anticancer therapy and is another example of a hydrophobic block often used for polymersome construction [
50,
51,
52].
The addition of charged polymers can influence the interactions of polymers with both therapeutic loads and biological compartments like cell membranes. They are widely used for the complexation of therapeutic nucleic acids and some examples will be explored in
Section 6. However, some positively charged polymers can have biological activity in their own right, as demonstrated in the example of PCL-poly(lysine-stat-(S-aroylthiooxime) polymersomes [
53]. PolyLys is a positively charged antimicrobial peptide [
54] that promotes bacterial death and cellular uptake via interactions with cell membranes. Incorporation within a copolymer provides increased peptide stability and at the same time serves as a hydrophilic block. The s-aroylthiooxime (SATO) component serves as a source of H
2S, which promotes cell migration and adhesion and has great potential in diabetic wound healing [
55]. Since SATO is hydrophobic, incorporation into a copolymer increases its solubility and provides good dispersibility. When administered as a wound dressing spray (layer thickness 375 nm), the vesicular morphology was preserved and the addition of cysteine led to gradual H
2S release. The combined effects of wound healing and bacterial inhibition observed in a murine model offer the potential for an efficient and simple platform for diabetic wound healing with good patient adherence.
Therefore, the combination of hydrophilic blocks with hydrophobic polymers and other functional structural elements can lead to the formation of novel polymersomes for API delivery.
1.2. Theranostic Polymersomes
One of the great conveniences of these nanosystems is the ability to introduce imaging/diagnostic tags that can be used in diseased cell/tissue imaging and/or to monitor their transport and distribution in the organism. In addition, they can also be used as therapeutic agents, like in photodynamic therapy, or can be combined with other types of APIs delivered by the same polymersome.
One example is the fluorescence introduced by aggregation-induced emission (AIE) [
56]. The low fluorochrome loading and fluorescence quenching upon aggregation are frequent phenomena associated with conventional fluorochromes. On the contrary, when luminogens are incorporated into the block copolymers, they aggregate and generate bright photoemission characterized by improved contrast and photo-stability, which enhance cell/tissue imaging [
8]. An example of this type of imaging polymersomes is poly(ethyleneglycol)-block-poly(caprolactone-gradient-trimethylene carbonate) (PEG-P(CLgTMC)) loaded with the photosensitizer BODIPY [
9]. Terminal blocks of tetraphenylethylene pyridinium allow preferential polymersome accumulation on the surface of mitochondria, a preponderant organelle for cell survival [
8].
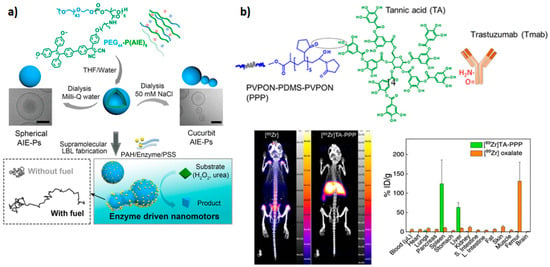
A recent study demonstrated that asymmetric cucurbit-shaped polymersomes could be used as nanomotors with a variety of potential applications, including drug delivery, cell/tissue imaging, or photodynamic therapy [
9]. In this case, cucurbit polymersomes based on PEGylated AIEgenic poly(trimethylene carbonate) blocks PEG
44-P(AIE)
5 were coated with catalytic urease-based machinery via a layer-by-layer (LBL) technique (
Figure 2a). When exposed to the fuel (urea), cucurbit polymersomes outperformed their spherical counterparts.
Figure 2. Examples of theranostic polymers: (
a) Schematic representation of cucurbit-shaped polymersome assembly and nanomotor properties upon exposure to appropriate fuel; represented with permission from [
9]. (
b) Assembly of poly(
N-vinylpyrrolidone)
5-
b-poly(dimethylsiloxane)
30-
b-poly(
N-vinylpyrrolidone)
5 polymersomes coated with tannic acid and decorated with
89Zr and antibody trastuzumab ([
89Zr]TA-PPP) through hydrogen bonding and ion pairing (top). Biodistribution of free
89Zr and [
89Zr]TA-PPP after 24 h. Reproduced with permission from [
57].
Chelator-free labeling with positron emission tomography (PET) radiotracers is preferable since it avoids chelator optimization and multistep modifications of the nanosystem. On the other hand, the incorporation of radiotracer within the hydrophilic cavity or hydrophobic layer of polymersomes can result in loss of tracer under physiological in vivo conditions. Coating of poly(
N-vinylpyrrolidone)
5-
b-poly(dimethylsiloxane)
30-
b-poly(
N-vinylpyrrolidone)
5 (PVPON
5-PDMS
30-PVPON
5) polymersomes with tannic acid provided a polyphenolic anchor layer for radiotracer
89Zr and antibody ligand trastuzumab for targeting HER-2-positive breast cancer [
57]. Although the system was not applied for tumor imaging in vivo, adsorption of the polymersome surface demonstrated a marked influence on biodistribution (
Figure 2b).
1.3. Polymersomes Decorated with Targeting Ligands
Polymersomes decorated with targeting ligands can improve their selectivity against specific targets, including immune and cancer cells, and could be used for intracellular API delivery, immunotherapy, or vaccines. An example of this approach is a PEGylated polymersome composed of PEG
22-
block-poly[(ε-caprolactone)
38-
gradient-(trimethylene carbonate)
37] (PEG-p(CL-TMC)) decorated with immunoglobulin fragment Fc and CpG oligodeoxynucleotide, that target Fc receptors and activate TLR-9 receptors located on the endosomal membrane, respectively [
20]. Surface PEGylation is a commonly used approach to improve the pharmacokinetic properties of nanosystems by decreasing interactions with immune system components such as the complement system and mononuclear phagocyte system [
58]. However, conjugation with immune-cell-targeting ligands greatly increased interactions with and the uptake by immune cells. Both ligands demonstrated a marked increase in polymersome interactions with immune cells. Nonetheless, when the combination of both ligands was applied, the CpG ligand was the main driving force of cellular interactions, while the synergistic effects of both ligands on immune system activation were observed. This study demonstrates that PEGylated polysomes like PEG-p(CL-TMC) could be developed for potential use as vaccines. In another example of nanocarriers labeled with two targeting ligands, PEG-poly(D,L-lactic-co-glycolic acid) (PLGA) polymersomes loaded with curcumin and decorated with neuron-specific transferrin and Tet-1 ligands demonstrated neuroprotection and improved cognitive function in a murine model of Alzheimer’s disease [
59].
To account for the limited amount of overexpressed receptors on the targeted cells and to prevent receptor saturation, one strategy is to include multiple ligands that target different receptors. This approach allows for the targeting of different cell populations in vivo [
60]. On the other hand, to avoid targeting non-tumor cells expressing the same receptors, a single multivalent low-affinity ligand that target different receptors can be used [
10]. This approach was used to improve the capacity of poly(2-(methacryloyloxy)ethyl phosphorylcholine)-poly(2-(diisopropylamino)ethyl methacrylate) (PMPC-PDPA) polymersomes to target the scavenger receptor class B member 1 (SRB1) and scavenger receptor class B member 3 (CD36) present on the surface of tuberculosis- and Staphylococcus aureus-infected macrophages or cancer cells [
10]. This targeting capacity is based on the interactions with the phosphorylcholine groups (PC) of the PMPC chains, which promote the internalization of polymersomes by endocytosis. Taking advantage of these polymersomes to target monocytes, their use in cancer immunotherapy can be promising, taking into account that these cells represent 2−8% of the blood cells.
A recent study by Tjandra et al. demonstrated the importance of ligand density optimization using the example of poly(ethylene glycol)-
block-poly(
N-isopropylacrylamide-co-perylene diester monoimide) (PEG
43-
b-P(NIPAM
21-
co-PDMI
9)) ellipsoid polymersomes [
61]. In this case, polymersome size (<100 nm or ~200 nm) did not influence cell uptake. However, a threshold density (four ligands per polymersome) of medulloblastoma cell (DAOY)-targeting ligand was required for enhanced uptake. Here, a highly specific targeting ligand, heptapeptide FSRPAFL, with high binding activity, was selected through a peptide phage display library. The non-linear tendency between ligand density and cell uptake was especially obvious in cells with many targeted receptors (DAOY compared to HEK) as the cumulative effect of interactions became much more pronounced. At the same time, the chosen heptapeptide provided stealth properties to the polymersomes, and reduced interactions with immune cells were observed when compared to control polymersomes.
Another study described a composite methyl-poly(ethylene glycol)-polylactide (mPEG–PLA)/tocopherol-polyethylenelglycol-succinate (TPGS)-lactobionic acid (TLA) polymersomes equipped with TLA ligands that target asialoglycoprotein receptor (ASGPR) in hepatocellular carcinoma cells [
11]. At the same time, this nanosystem hampers multidrug resistance by inhibiting P-glycoprotein, leading to improved internalization and intracellular accumulation of drugs. The iRGD peptide is also often used to decorate polymersomes and improve their binding to tumor vascular cells and selective internalization for targeted drug delivery [
62,
63].
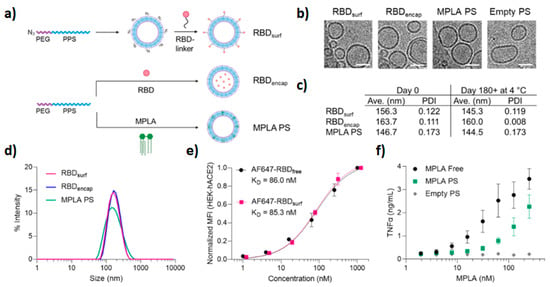
The pandemic caused by SARS-CoV-2 elicited a rapid response and the development of vaccines. Although nucleic-acid-based nanovaccines have been approved for clinical use so far, subunit vaccines based on spike protein and its subdomains are also being developed. To improve humoral and cellular immunogenicity towards the receptor binding domain (RBD) of spike protein, Volpatti et al. prepared, as a new generation of vaccines, oxidation-responsive poly(ethylene glycol)-
bl-poly(propylene sulfide) (PEG–PPS) polymersomes for delivery of antigen and adjuvant [
64]. To evaluate the ability of PEG-PPS to activate B cells and induce the production of RBD antibodies, polymersomes were decorated with RBD (RBD
surf) or encapsulated RBD in the polymersome cavity (RBD
encap), and were supplemented with shell-encapsulated adjuvant monophosphoryl-lipid-A PS (MPLA PS). In vivo studies in the murine model demonstrated that only adjuvanted RBD
surf produced neutralizing IgG and RBD-specific germinal center B cells. Both surface and encapsulated RBD stimulated T cells (CD
4+ and CD
8+) and Th
1-type cytokines. Furthermore, antigen/adjuvant-loaded systems are stable for months at 4 °C and can be loaded with multiple antigens (
Figure 3).
Figure 3. Stable polyfunctional polymersomes for delivery of antigens: (
a) schematic of polymersome preparation and loading. Empty and RBD-loaded polymersomes were prepared using the thin film method followed by extrusion, while MPLA polymersomes were fabricated by flash nanoprecipitation followed by extrusion. (
b) Cryoelectron microscopy of prepared polymersomes (scale 50 nm). (
c,
d) Respective size/size distribution curves and PDI of polymersomes. Size after six months is also indicated. (
e) Conjugation of RBD to the polymersome surface does not influence RBD (evaluated by RBD ACE-2 receptor binding on normal human cells). (
f) Dose-dependent secretion of TNFα when stimulated by MPLA. Reproduced with permission from [
64].
1.4. Stimuli-Responsive Polymersomes
Tailoring of polymer structural properties enables the construction of stimuli-sensitive polymersomes in order to regulate the payload release upon exposure to various internal/physiological or external stimuli (
Figure 1d). Furthermore, dual-stimuli-responsive polymersomes can be constructed, such as photo- and redox-responsive polymersomes [
65], temperature- and pH-responsive polymersomes [
7], or light- and reduction-responsive polymersomes [
4] able to respond to two types of stimuli in order to improve the efficiency of treatment. A variety of polymersomes comprised of copolymers with characteristics that influence the response to stimuli has been described so far, resulting in pH-, temperature-, enzyme-, oxygen-, light-, reduction-, or hypoxia-responsive polymersomes, as described below. There are infinite approaches to take advantage of the characteristics of different extra- and intracellular disease environments and apply the stimulus for targeted release of drugs from the vesicles. Additionally, they can be combined with other functionalities already discussed in this section [
66].
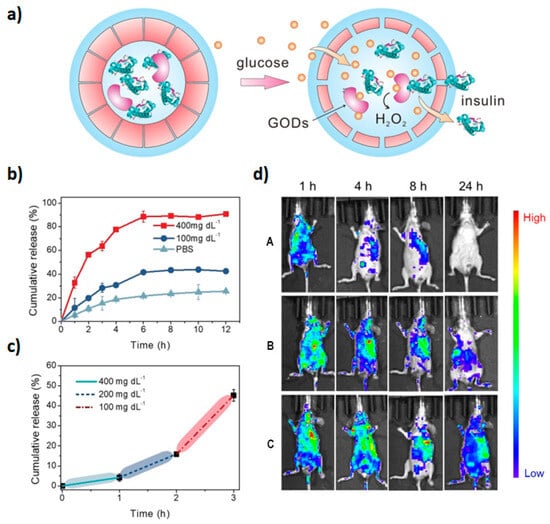
Taking advantage of the fact that reactive oxygen species (ROS) are overexpressed in a variety of diseases, ROS-responsive polymersomes could be promising diagnostic and treatment tools. Inspired by physiological mechanisms that control the permeability of cell and organelle membranes, Zhen et al. applied an innovative concept to prepare synthetic polypeptide polymersomes composed of PEGylated poly(L-cysteine) connected to hydrophobic cholesterol moieties via thioether bridges [
67]. Upon exposure to oxygen species, a transition from β sheet to α helix occurs and results in vesicle thinning, enabling the release of an encapsulated load. In the case of incorporated hydrophobic compounds, increased hydrophilicity of oxidized sulfur species and modified crystallinity of the cholesterol layer decrease interactions with the load and promote the release. On the other hand, the changes in the pattern of hydrogen bonds are likely responsible for the transport of hydrophilic species captured within the hydrophilic cavity. The ability of such nanoreactors to deliver therapeutic proteins was explored via the incubation of insulin- and glucose oxidase(GO)-loaded polymersomes with glucose. H
2O
2 produced by the reaction of GO and diffused glucose triggered vesicle permeability and the release of functional insulin (
Figure 4).
Figure 4. Release of insulin upon glucose stimulation: (
a) glucose influx triggers H
2O
2 synthesis by glucose oxidase which, in turn, induces vesicle permeability and insulin release; (
b) insulin release kinetics under normal and hyperglycemic conditions (100 vs. 400 mg dL
−1); (
c) cumulative insulin release under increasing glucose concentrations; (
d) biodistribution of insulin within 24 h of administration in diabetic animals ((A) free insulin; (B) vesicular insulin) and normoglycemic animals ((C) vesicular insulin). Reproduced with permission from [
67].
Biodistribution studies have revealed slower clearance of encapsulated insulin (>24 h) when compared to free insulin (4 h) in diabetic mice, but faster than in normoglycemic animals, confirming in vitro observations of glucose-triggered insulin release. Entrapped insulin was also able to maintain prolonged normal glucose levels when compared with free insulin (4 h vs. 1 h, respectively), as well as higher blood insulin levels.
Reduction-responsive polymersomes have also been described. Taking advantage of the presence of glutathione (GSH) in the cytosol, the release of encapsulated cargos from GSH-responsive polymersomes can be triggered. It can be achieved by the presence of disulfide linkage in the copolymers, as they break down in response to reducing agents [
68]. Alternatively, GSH-sensitive disulfide bridges can be used for the conjugation of drugs [
69,
70] or sulfur-containing prodrugs for the delivery of nitric oxide [
71]. Recently, disulfide moieties were used to crosslink hydrophobic layers of polymersomes and enhance drug release after intracellular degradation [
72].
A novel vesicular system described by Cheng et al. exploited the heterogeneous distribution of redox potential (ROS
extracellular 0.1–1 mM vs. ROS
intracellular 1–10 μM and GSH
extracellular 2–20 μM vs. GSH
intracellular 10 mM) for sequential delivery of hydrophilic (Dox.HCl) and hydrophobic (paclitaxel Ptx) drugs to cancer cells [
73]. Extracellular release of hydrophilic drugs occurs via the oxidation of cystine bridges that connect the PEG and PCL blocks of the copolymer. The resulting sulfoxide and sulphone groups change the hydrophilicity and reactivity of the cystine bridges and make them more susceptible to subsequent degradation by intracellular GSH, leading to Pxt release.
Associating light and reduction-responsive block copolymers to PEG, such as poly-coumarin-based disulfide-containing monomer (PCSSMA), gave rise to a dual-stimuli-responsive PEG-b-PCSSMA-based polymersome, where disulfide linkage shows reduction-responsive characteristics and coumarin groups are photo-responsive [
5]. Upon irradiation with visible light (430 nm), the generation of reactive primary amine groups cross-linked the membranes due to the amidation reactions, leading to the cleavage of coumarin moieties. The polarity reversal in the membrane allowed the release of encapsulated small molecules. The bigger molecules were released later, upon disassembly of the cross-linked vesicles provoked by incubation with GSH, which is abundant in the cytosol of cancer cells. In this way, these dual-responsive polymersomes could sequentially release different therapeutic agents.
Ph-responsive polymersomes are promising as drug delivery systems due to their potential to release the drug at the desired site depending on pH value differences [
74]. Their membranes can change the conformation between swelling and shrinking according to the acidic and normal physiological pH, respectively. While protection of cargo is provided under normal physiological pH, the control of the drug delivery in an acidic tumor environment and increased selectivity of the drug action are enabled at lower pH characteristics of the tumor microenvironment [
74]. Furthermore, at certain pH values where polymersomes are only semi-swollen, it is possible to reach a retarded release profile [
12].
A hydrophobic component that is often used is a pH-sensitive poly(2-(diisopropylamino)ethyl methacrylate) (PDPA), and it was concluded that PEG-PDPA polymersomes with Dox significantly reduced drug toxicity and improved cancer therapy in a zebrafish embryo tumor model [
75].
The assembly of a new type of polyphosphazene polymersome with a pH-sensitive ortho ester group was assisted by π–π interactions of benzene rings and Dox [
76].
On the other hand, temperature-responsive polymersomes are susceptible to elevated temperature in a tumor environment. Increased tumor metabolism or externally controlled temperature can trigger drug release when polymer blocks with appropriate critical solution temperature (LCST) are used [
13]. Vesicles composed of biocompatible triblock polymer poly(
N-vinylcaprolactam)
n-
b-poly(dimethylsiloxane)
m-
b-poly(
N-vinylcaprolactam)
n-(PVCL
n-PDMS
m-PVCL
n) experience reversible decrease in size and membrane thickness in the range of 37–42 °C due to favorable LCST of the PVCL block [
77]. Sustained Dox release under physiological tumor conditions (37–42 °C and low pH) was assisted by the presence of pH-sensitive linkers. Similar effects were observed in the case of mPEG-
b-PNIPAM-
b-P(DEAEMA-
co-BMA) triblock copolymer with thermosensitive poly(
N-isopropylacrylamide) (PNIPAM) and pH-sensitive hydrophobic poly[2-(diethylamino)ethylmethacrylate (PDEAEMA) segments [
7]. 2-hydroxy-4-(methacryloyloxy)benzophenone] (BMA) represents a photo-crosslinker for crosslinking the hydrophobic core for increased systemic stability [
7]. Hydrophilic Dox and hydrophobic Ptx were encapsulated in respective polymersome compartments and were released in a controlled manner under tumor conditions (42 °C and pH < 7).
Considering the overexpression of enzymes involved in pathological conditions, enzyme-responsive drug delivery systems can be very promising in cancer and infection therapies because their responsivity is very selective against specific targets. The substrate of the enzyme is integrated into the polymer and, thus, when enzymes cleave the substrate, the polymersome structure destabilizes, allowing the release of the encapsulated drug. The presence of enzymes at specific sites is of particular interest, causing drugs to be delivered only to a specific location. For example, PEG-PLA bridged with a synthetic polypeptide PVGLIG sensitive to matrix metalloproteinase 2 (MMP2) overexpressed in tumor extracellular matrix exhibited a seven-fold increase in SN38 release compared to the control formulation [
24].
In a study by Yao et al., the site-specific photodynamic (PDT) and imaging potential of polyacrylate-based polymersomes was triggered by NAD(P)H:quinone oxidoreductase isozyme 1 (NQO1) endogenous in some cancer cells [
78]. Polymer functionalized with quinone trimethyl locks was conjugated with photosensitizers Nile blue and coumarin. Without the stimuli, the theranostic function was locked by aggregation and quinone-photoinduced electron transfer quenching. After cellular uptake and NQO1 exposure, self-immolative photosensitizer release and activation were induced. An additional functional element, targeting the ligand cRGD, increased cellular internalization and contributed to the overall in vitro and in vivo cytotoxic effects upon near-infrared (NIR) irradiation of Nile blue vesicles (~90% tumor growth inhibition). In polymersomes conjugated with a nuclear magnetic resonance (MR) imaging agent, gadolinium-tetraazacyclododecanetetra acetic acid (Gd-DOTA), the moieties liberated after self-immolative quinone release led to intra/inter-polymer cyclization followed by the release of Gd-DOTA. When Gd vesicles were loaded with hydrophobic camptothecin (Cpt) and hydrophilic Dox.HCl, a dramatic increase in drug release was observed upon NQO1 exposure (>90% against <20% without stimulus). Both Nile blue and Gd-DOTA polymersomes were successfully applied for tumor imaging in vivo.
The release of photosensitizer pyropheophorbide a (Ppa) and lapatinib (Lap) from poly(oligo(ethylene glycol) methyl ether methacrylate) (pOEGMA) polymersomes is another example of enzyme-sensitive vesicles [
79]. Here, Ppa and pOEGMA were bridged by a cathepsin B-sensitive peptide linker. Embedding of dendritic Ppa into the cell membrane facilitates cell and tumor penetrability, and ROS produced by laser irradiation combined with Lap to generate synergistic in vivo antitumor effects.
Hypoxia-responsive polymersomes can also be used following the same principle. Considering that treatment of triple-negative breast cancer (TNBC) is challenging because of rapid cell proliferation and inadequate blood flow which decreases oxygen concentration at the tumor site, it is possible to release the drug at the exact location of the tumor by constructing a hypoxia-responsive polymersome. PLA-PEG polymer with diazobenzene linker reduced under hypoxic conditions, leading to the disintegration of the polymersome membrane [
62]. At the same time, the iRGD peptide integrated into the membrane directed them to penetrate solid tumors, as iRGD interacts with specific integrins present in endothelial cancer cells and promotes transcytosis extravasation [
62].
In light-responsive polymersomes, the release of encapsulated drugs is achieved by exposing polymersomes to a photo source with a determined wavelength, allowing localized and non-invasive treatment in a specified period of time [
80]. This approach enables intensive control and direction of the treatments.
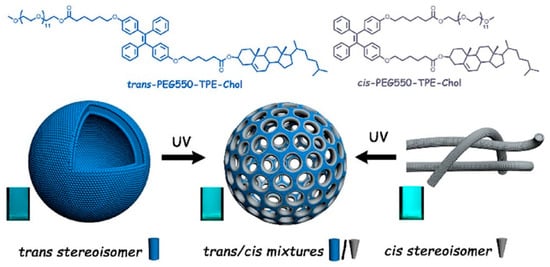
The addition of hydrophobic tetraphenylethene stilbene-type motifs (TPE) to the hydrophobic cholesterol core into PEG-TPE-Chol enables the formation of membrane pores [
48]. The shape of the self-assembled aggregates based on PEG-TPE-Chol depends on the configuration of the used TPE copolymer [
48]. For example, when trans-configuration was used, spherical vesicles were formed, while the cis-configuration of the same block copolymer yielded cylindrical micelles. However, the mixture of PEG-TPE-Chol isomers (trans/cis = 60/40) resulted in vesicles with porous membranes (
Figure 5) during the self-assembly in water. Moreover, trans−cis photoactivated isomerization can occur under UV illumination and can make the self-assembly photo-responsive. Under high-intensity UV light, vesicles and micelles (formed by trans-PEG-TPE-Chol and cis-PEG-TPE-Chol) tend to form porous vesicles [
48].
Figure 5. Example of a highly functionalized hydrophobic block used for the formation of light-gated polymersomes. Reproduced with permission from [
48].
2. Recent Advancements in Polymersomes as Drug Delivery Systems
The use of polymersomes as drug delivery systems is promising because it takes advantage of their tunable stability, selective permeability, sustained drug release, and targeted delivery [
114]. Therefore, keeping in mind the characteristics of polymersomes, they can be used in a variety of applications, and some of them will be described below.
2.1. Chemotherapy
Chemotherapeutic agents are associated with poor efficacy against cancer cells due to low systemic stability and resistance development but also with off-target toxic effects. Incorporating them into vehicles can improve the pharmacokinetic profile of systemically administered drugs and direct them to the specific sites of action, overcoming adverse side effects in the process [
75].
For example, Dox leads to the progressive development of heart failure, causing irreversible damage in the cardiac muscle and dysregulation of the immune system in dose- and treatment-time-dependent ways [
42]. The photosensitive
o-nitrobenzyl (ONB) linker was combined with redox-sensitive disulfide in poly(ε-caprolactone)-ONB-SS-poly(methacrylic acid) (PCL-ONB-SS-PMAA) [
65]. Polymersomes loaded with UCNP and Dox completely disintegrated after laser irradiation (30 min at 980 nm), while exposure to 5 mM GSH led to decomposition within 15 min. Cumulative effects of GSH and irradiation on Dox release were observed. Synergistic anticancer effects were observed in vitro and in vivo after irradiation. Additionally, UCNP luminescence was also applied for imaging in nude mice bearing the A549 lung tumor xenograft. A biodistribution study showed that accumulation in tumor and liver gradually declines after the first day, but is still present four weeks later.
An example of a promising therapeutic application of polymersomes was observed in colorectal cancer, one of the most fatal cancers worldwide [
115]. 7-ethyl-10-hydroxy camptothecin (SN38), which is a more potent active metabolite of the well-known irinotecan (Topoisomerase-1 inhibitor), has compromised clinical application due to low solubility and poor stability, as it is converted into its inactive form under physiological pH. Enzyme-responsive polymersomes can be promising options for targeted drug release when exposed to enzymes that are overexpressed in particular cancer tissues. PEG-PLA linked through a cleavable synthetic peptide sequence, PVGLIG, were prepared for targeting the tumor-associated MMP-2 enzyme, allowing a seven-fold higher release rate than when exposed to the MMP-2 enzyme at physiological conditions [
24]. On the other hand, the addition of targeting DNA AS1411 aptamers yielded a guided drug delivery against nucleolin-positive cells. The in vitro study demonstrated an increased cytotoxicity of Apt-SN38-peptide-polymersomes against C26 cancer cells. A PEGylated surface that hampers the detection of nanoparticles by the mononuclear phagocyte system (MPS) and prolongs their half-life time in the bloodstream, as well as their small size (<200 nm) enables their uptake via the EPR effect. Their more specific and efficient drug delivery allowed increased tumor accumulation and penetration, enhanced cellular uptake via receptor-mediated endocytosis, and presented a promising tool to improve the efficacy and safety of cancer chemotherapy. Moreover, in vivo studies also demonstrated high therapeutic rates for polymersomes with cleavable peptide sequences and even higher rates for polymersomes with targeting ligands on their surface.
In some cases, self-aggregation into polymersome structures can involve using organic solvents or increased temperatures incompatible with biologicals. The copolymer poly(3-methyl-
N-vinylcaprolactam)-
b-poly(
N-vinylpyrrolidone) (PMVC
58-PVPON
65), composed of hydrophilic polyvinyl derivatives assembles in water at decreased temperatures (T < 20 °C) [
42]. Control over lower critical solution temperature (LCST) and aggregation properties was obtained via hydrophobic modifications of the PVPON component and copolymer composition. The model drug (Dox) was loaded at room temperature without the use of organic solvents with exceptional efficiency (EE 95%, LC 49%, 360 nm). Another highlight of this copolymer system is its resiliency against physiological conditions, and very low Dox release was observed at pH 7.4 and 5 during 24 h. Moreover, the presence of poly(vinylpyrrolidone) in the outer polymersome corona increased the hydrophilicity of the vesicles resulting in a good in vitro stability in serum. The in vivo stability of PMVC
58-PVPON
65 and its ability to attenuate Dox toxicity was evaluated in vivo by administering a toxic non-survival dose to mice (15 mg kg
−1 week
−1) and was compared to liposomal Dox. Although the authors did not provide the liposomal composition, both formulations provided 14-day survival, albeit some toxicity was observed with liposomal Dox. The mild encapsulation conditions make this formulation promising for the delivery of sensitive chemo and biological therapeutics. Nonetheless, the introduction of stimuli-sensitive motives will be needed to enable the release of therapeutics in order to broaden the therapeutic applicability.
In another recent example, in the Ptx-loaded PEG-PCL polymersomes, more attenuated and prolonged release of the drug was observed under pH5 than at pH7, presumably due to more gradual hydrolysis degradation of the polymer, but also with the possible contribution of aggregated state of Ptx within the PCL layer [
86]. In an orthotopic syngeneic animal model of glioblastoma tumor, polymersome Ptx demonstrated greater tumor decrease and eradication than free Ptx or clinically approved liposomal formulation (Lipusu
®).
Bacterial keratitis or acute ophthalmic infection is another disease characterized by elevated ROS levels, and alleviation of oxidative stress can be combined with antibiotics to mediate the infection. Block copolymer poly(polyethylene glycol methyl ether methacrylate)-
co-
N-benzylacrylamide)-
block-poly(2-methylthioethyl methacrylate) (P(PEGMA
10-
co-PBA2)-
b-PMTEMA
25) with ROS scavenging thioether fragments was combined with mucoadhesive and bacteria-targeting phenylboronic acid motives for in vivo delivery of antibiotic ciprofloxacin hydrochloride [
116].
Combination therapy is based on concurrent targeting of different disease mechanisms by using two or more chemotherapeutic agents [
117]. The improved therapeutic efficacy results from reduced required dosage of different drugs compared to monotherapy, decreased risk of side effects and drug resistance associated with therapy [
26,
118]. The concept was used for targeted co-delivery of Dox and camptothecin (Cpt) to non-small-cell lung cancer (NSCLC) by hyaluronic acid-
b-polycaprolactone polymersomes [
26]. Hyaluronic acid (HA) is biocompatible and biodegradable, and can be used as a targeting agent due to interactions with tumor receptors that are overexpressed in many tumors of epithelial origin. To improve accumulation in cancer cells, it was conjugated with a FOXM1 DNA aptamer. Drugs were loaded with high efficiency in both mono and co-loaded monodispersed polymersomes (140–170 nm; Dox EE 80% and Cpt EE 55%, LC 4–6% for all formulations). It was demonstrated that 24% of Dox and Cpt were immediately released and the rest was released in a sustained manner. The ability of HA to target CD44 receptors resulted in cellular uptake by endocytosis and was complemented by the ability of FOXM1 to downregulate efflux pumps. Increased cellular accumulation of drugs exerted synergistic in vitro activity against NSCLC by inducing apoptosis. Moreover, in vivo tests in the heterogeneous heterotopic SK-MES-1 murine model showed a significant tumor suppression effect [
26].
Hydrophilic Dox and hydrophobic Ptx encapsulated in mPEG-
b-PNIPAM-
b-P(DEAEMA-co-BMA) polymersomes demonstrated efficient cellular uptake and a synergistic cytotoxic effect in human cervical HeLa and breast MCF-7 cancer cells [
7]. The L929 cell line derived from normal subcutaneous tissue was used as a model of healthy cells. The absence of a cytotoxic effect demonstrated a lack of drug release under normal physiological conditions (pH 7.4 and 37 °C), confirming the sensitivity of the copolymer to the characteristic cancer cell microenvironment (pH 6.0–7.0 and 42 °C). Synergistic effects were also observed for combining these two drugs delivered by Pluronic FA-F127-PLGA/PLGA-F127-PLGA polymersomes to ovary carcinoma OVCAR-3 cells [
119].
In another recent example of combination therapy, PLA-HA polymersome delivered Dox with peptide melittin. In multidrug-resistant MCF-7 cells, melittin suppressed efflux pumps and showed synergistic cytotoxic effects in combination with Dox [
120].
2.2. Immunotherapy
Immunotherapy has been studied as a weapon for treating or retarding the progression of tumors, being used for long-term curative effects [
25]. The use of polymersomes in vaccine formulations brings new perspectives on antigen protection, controlled drug release, elevated antigen density, and co-delivery of different components to the same cell [
20].
Decorating polymersomes with Fc fragment and CpG ODN (CpG oligodeoxynucleotide is an example of a potential vaccine, and has already been discussed in
Section 3.3 [
20]. Other studies have investigated the potential use of polymersomes in antigen vaccines for tumor immunotherapy [
121,
122,
123]. Tumor-specific antigens or tumor-associated antigens, which can be proteins or peptides, are processed by antigen-presenting cells (APCs), and activate T cell-mediated immune responses. As their application is associated with some limitations, such as poor immunogenicity, biocompatibility, cellular uptake, and fast elimination from the bloodstream, a delivery system can also be advantageous. For that, the drug delivery system must allow adequate protein release and maintain the stability of proteins. Oncolytic peptide LTX-315 is able to induce immune responses and tumor regression. Xia et al. designed a cyclic peptide cRGD-functionalized chimeric polymersomes for delivery of the LTX-315 and the immunoadjuvant CpG to be used in immunotherapy against malignant B16F10 melanoma [
25]. Polymersomes loaded with positively charged LTX-315 were assembled from PEG-P(TMC-DTC)-poly(aspartic acid) (PEG-P(TMC-DTC)-PAsp) which provided a negatively charged inner shell surface [
124], and cRGD-decorated PEG-P(TMC-DTC) [
125]. To encapsulate CpG, PEG-P(TMC-DTC) conjugated to positively charged spermine was used [
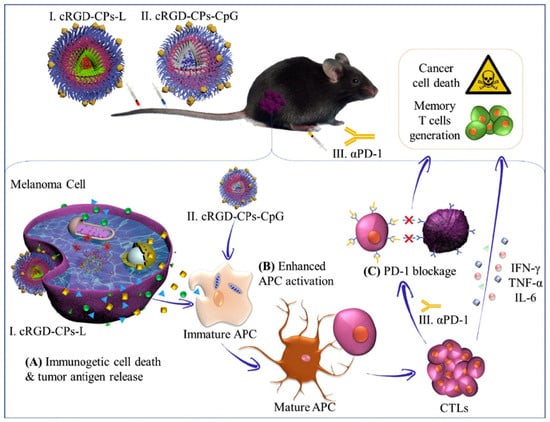
126]. The in vivo studies demonstrated that systemic administration of cRGD-functionalized polymersomes with LTX-315 peptide, combined with the immunoadjuvant CpG-loaded polymersomes and with anti-PD-1 antibody, resulted in a strong immune response (increasing CD8+, cytotoxic T lymphocytes, helper T cells, reducing T regulatory cells in the tumor, and increasing cytokines, such as IL-6, TNF-α, and IFN-γ), and long-term immune memory protection due to an increase in memory T cells (
Figure 6) [
25]. Systemic instead of intratumoral delivery of functional LTX is a great improvement of this type of therapy and could be broadened to treat metastases or inaccessible tumors.
Figure 6. Intravenous administration of polymersomes loaded with oncolytic peptide LTX-315 (cRGD-CPs-L) and immunoadjuvant CpG (cRGD-CPs-CpG): (
A) LTX-315 induces release of various tumor antigens, which in combination with polymersomal CpG activate maturation of antigen prese nting cells (
B) and stimulation of T cell response; (
C) Addition of αPD-1 antibody enhanced immunotherapeutic effect by PD-1 blockage and contributed to generation of long-term immune memory. Reproduced with permission from [
25].
2.3. Nucleic Acid Delivery
Biopharmaceuticals or biologics represent a large fraction of recently approved therapeutics composed of amino acids or nucleotides, and include hormones, antibodies, deoxyribonucleic acid (DNA), and diverse ribonucleic acids (RNAs) [
27,
127]. Despite their potency and specificity, some factors compromise their therapeutic efficacy, including structural complexity, poor stability, immune cell activation, low membrane permeability, and in vivo clearance, leading to low intracellular delivery and short circulation half-lives (sometimes in minutes). Nanocarriers such as polymersomes can protect them and prolong their circulation time, promote targeted delivery and cellular internalization, and improve their capacity to exert the therapeutic effect effectively and safely [
27,
127,
128]. Nevertheless, problems related to preparation and encapsulation have been described, including insufficient loading and encapsulation efficiency, as well as drawbacks associated with the instability of biologics during the process. With respect to this, new preparation methods have emerged to overcome these limitations (
Section 5).
Non-coding RNAs like silencing siRNA are strong tools for antitumor therapy, albeit with drawbacks associated with safety, efficiency, instability, and lack of targeting capacity. Thus, polymersomes are good candidates for targeted RNA delivery [
129,
130].
The use of biodegradable, non-ionic copolymers in polynucleotide delivery could circumvent undesired cytotoxic contributions by the positively charged components. Poly(
N-vinylpyrrolidone)-
b-poly(dimethylsiloxane)-
b-poly(
N-vinylpyrrolidone) (PVPON
14−PDMS
47−PVPON
14) is the first example of non-ionic polymersomes for siRNA delivery [
131]. Polymersomes prepared by the thin film method (<105 nm) were in this case smaller (20%) than those prepared by nanoprecipitation, but this was attributed to the use of the additional extrusion step. Polymersomes loaded with PARP1 siRNA and labeled with the Cy5.5 imaging tag significantly reduced targeted protein levels and suppressed human breast cancer MDA-MB-361TR and murine 4T1 proliferation by 35% after a 6-day exposure. In a syngeneic murine model, a four-fold increase in animal survival was observed against the control animals after three months (80% vs. 20%, respectively).
The compartmentalized nature of polymersomes was exploited for the co-delivery of tumor growth factor β siRNA (siTGF-β) and shikonin (SK) [
132]. The combined immunotherapeutic effects of siTGF-β and SK loaded into a hybrid nanoassembly of PEI-PCL and FA-decorated 1,2-distearoyl-
sn-glycero-3-phosphoethanolamine-
N-polyethyleneglycol led to immunogenic cell death and infiltration of cytotoxic T lymphocytes in a syngeneic orthotopic in vivo model of triple-negative breast cancer. At the same time, the formation of lung metastasis was suppressed, and long-term antitumor memory delayed the growth of secondary tumors.
Highly functionalized polymersomes for the delivery of retinoblastoma-binding protein 4 siRNA and temozolomide were assembled from diblock Angiopep-2-PEG-
block-poly-(2,2,3,3-tetrafluoropropyl methacrylate) and triblock PEG-
block-poly-(2,2,3,3-tetrafluoropropyl methacrylate)-
block-poly[(
N-(3-methacrylamidopropyl) guanidinium)]. Here, positively charged guanidinium moieties were used for siRNA complexation. The addition of fluorine increases the hydrophobicity and stability of the membrane and has previously been associated with increased efficacy of polyplexes [
133]. Efficient crossing of BBB mediated by the targeting ligand Angiopep-2 led to a synergistic therapeutic effect in a transgenic orthotopic model of glioblastoma [
134].
2.4. Protein Delivery
A similar strategy based on the derivatization of hydrophilic sides with ionizable groups promotes the encapsulation of large amphoteric/amphiphilic proteins and was already presented for the delivery of nucleic acids or immunogenic factors. Granzyme B was loaded into PEG-
b-poly(trimethylene carbonate-
co-dithiolane trimethylene carbonate)-
b-spermine for multiple myeloma therapy [
126]. Self-assembly by nanoprecipitation was accompanied by self-crosslinking and was followed by decoration with HA (HA-RCLP-GrB). Under reductive conditions, 80% of the protein was released within 24h. Formulation with optimized HA density (30%) induced apoptosis in vitro, and alleviated osteolysis in an orthotopic murine mode with prolonged survival time.
HA-PLA loaded with β-galactosidase was applied for enzyme replacement therapy (ERT) in a lysosomal storage disorder, GM1 gangliosidosis. Successful uptake of polymersomes by diseased fibroblasts promoted healthy autophagic activity [
97].
Oral delivery is the preferable non-invasive administration route from the perspective of patient adherence. However, it is not applicable for big hydrophilic molecules sensitive to the harsh environment of the digestive tract and poor epithelium adsorption. ABA copolymer PEG
5-poly(propylene)
68-PEG
5 (poloxamer 401, Pluronic L-121) polymersomes have the ability to transfer large molecules like proteins across biological barriers and could be potentially used to cross endothelial intestinal barrier for systemic delivery of APIs or to treat intestinal inflammation [
135]. Polymersomes loaded with the model IgG-FITC antibody (Ab) or therapeutic adalimumab were prepared by co-dissolution in PBS followed by purification by size exclusion chromatography and extrusion (~200 nm, PDI < 0.2). Loaded polymersomes demonstrated potential for intestinal permeation evaluated in a CaCo2 model and exerted anti-inflammatory activity by lowering TNFα levels in lipopolysaccharide-activated macrophages (murine J774A.1). The attenuation of TNFα levels observed at the basolateral side of a co-culture CaCo2/macrophage model points to successful release of Ab from the polymersomes, suggesting that the system could be applied for oral Ab delivery across the intestinal epithelium and subsequent release in the subepithelial compartment. However, the polymer concentration needed to deliver therapeutic levels of Ab was high enough to cause cell death and increase TNFα levels, raising the question of whether this polymer combination is the most appropriate delivery system.
A quality-by-design approach was used recently to develop L121/catalase for topical UV damage repair [
136]. Polymersomes were able to penetrate into viable epidermis and dermis after topical application and decreased lipid peroxidation upon UV irradiation, making them an interesting system for dermal therapy [
137].
Although protein delivery by polymersomes is usually related to their encapsulation within the hydrophilic internal area, Geervliet et al. decorated the surface of 2-(
N,
N’-diethylamino)ethyl methacrylate-based polymersomes with therapeutic collagenase type 1 (matrix metalloproteinase-1 MM1) for early liver fibrosis treatment [
138]. Polymersomes exhibited good pH, osmotic and shear-force stability, and preserved enzyme activity after optimization of purification and storage conditions. Limited release of MM1 from polymersomes indicates that a high degree of non-covalent interactions was established and that most of the MM1 was embedded within the polymersome membrane. When applied in vivo, MM1 vesicles inhibited collagen-I formation and inflammation, thereby alleviating early liver fibrosis symptoms.
Most of the formulations presented in this overview were prepared for administration as individual polymersomes, but they can also be embedded within different matrices. For example, hybrid polymersome–hydrogel composites [
139] and polymersome-loaded microneedle patches [
140] have been described. In a recent study by Edmans et al., glycerol monomethacrylate (GMA) and hydroxypropyl methacrylate (HPMA) polymersomes loaded with F(ab) antibody fragments were electrospun into bead-on-string mucoadhesive PEO nanofibers for buccal administration [
141]. Polymersomes maintained the morphology and F(ab) functionality during the electrospinning process, and efficient penetration of released polymersomes into the epithelium was observed in reconstructed human oral epithelia.
2.5. Photodynamic Therapy
The incorporation of porphyrin photosensitizer into the dextran backbone exploited π–π interactions to assemble photosensitive polymersomes. Dextran was additionally grafted with diazo and β-cyclodextrin (βCD) moieties, which contribute to cohesive forces via host–guest interactions. Upon UV radiation, diazo-βCD dissociates with an increase in size (from 200 to 250–300 nm) and surface morphology changes, which suggest a rearrangement of corona chains and could be used for on-demand drug release. Porphyrin photo-irradiation produces singlet oxygen, which is toxic for tumor cells [
142].
The presence of BODIPY photosensitizer in PEG-P(CLgTMC) polymersome allows a spatiotemporal control of the treatment, and it was concluded that the presence of a photosensitizer leads to ROS production in the presence of NIR irradiation light, and results in cancer cell apoptosis. The formulation was administered intratumorally and remained localized for at least 96 h, and tumor growth inhibition was observed upon NIR light irradiation [
8].
In another study, the photosensitizer used was rose bengal, a dianionic fluorescent dye with a potential application in anticancer therapy through the generation of singlet oxygen when exposed to irradiation [
12]. However, it has some disadvantages, such as a short half-life, a tendency to form aggregates, as well as a negative charge, which hampers cellular uptake. Therefore, polymersomes can be promising in allowing its protection, increasing its solubility, and directing its transport to the exact action site. Studies in carcinoma cell lines observed a slight increase in drug uptake by the cells, probably because of an almost neutral polymersome surface charge. Moreover, increased intracellular ROS generation and higher toxicity than for free rose bengal were observed [
12].
2.6. Sonodynamic Therapy
Sonodynamic therapy can be an alternative to photodynamic therapy since they act similarly but with different triggers. While photodynamic therapy depends on lasers, sonodynamic therapy depends on ultrasound [
143]. The mechanism of the last one involves the activation of a sonosensitizer that can transform oxygen into ROS, and damage nucleic acids and proteins in the cells, leading to their apoptosis. The advantages over photodynamic therapy include deeper and less invasive tissue penetrability, making it suitable for use in cancers located deep in the body [
144].
A stable PLA-PEG-based polymersome capable of loading both sonosensitizer verteporfin (Vp) and Dox (>95%) with high efficiency has been developed [
144]. Upon efficient intracellular uptake of both Dox and Vp by endocytosis, efficient production of ROS was followed by increased cytotoxic effect in response to ultrasonic energy. Moreover, the accumulation of polymersomes in the tumor site via the EPR effect was confirmed, evidencing selectivity for the tumor site. Finally, these polymersomes revealed no severe cytotoxicity in the body. Combined therapy was more effective in vitro and in vivo relative to single therapy [
144].
This entry is adapted from the peer-reviewed paper 10.3390/ma17020319