Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Physics, Condensed Matter
Magnetic fluids were historically the first active nano-dispersion material. Despite over half a century of research, interest in these nano-objects continues to grow every year. This is due to the impressive development of nanotechnology, the synthesis of nanoscale structures, and surface-active systems.
- magnetic fluids
- smart materials
- magnetic field
- feffofluids
- magnetic liquids
1. Introduction
The field of magnetic soft materials represents a burgeoning interdisciplinary scientific discipline that has emerged in recent decades. It encompasses tasks primarily associated with condensed matter physics, magnetism, magnetic hydrodynamics, inorganic and organic chemistry, colloid chemistry, computational and computer modeling, acoustics, engineering, and applied sciences. Magnetically soft materials encompass magnetic fluids, magnetic elastomers, and magnetic gels containing magnetic nanoparticles, all susceptible to modification via various magnetic and non-magnetic inclusions.
Historically, magnetic fluids (MFs) took the lead, constituting a colloidal system of magnetic material-coated nanoparticles dispersed in a carrier liquid. Magnetic fluid harbors single-domain superparamagnetic nanoparticles, typically around 10 nm in size. These early materials are considered the pioneers of ‘smart’ nano-dispersed materials [1,2], which are extensively explored in the scientific literature [3,4,5] and applied across various devices and technologies [6,7,8,9]. Magnetic fluid has a unique combination of fluidity and the ability to respond to an external magnetic field and has found an application in seals [7], controlled shock absorbers [6], various sensors [8], and acoustic systems [9]. The remarkable progress in nanotechnology and the ability to synthesize materials and structures over the past few decades has enabled the conceptualization of magnetic fluids as multiphase systems. In this context, magnetic nanoparticles function as distinct elements with a controlled structure that can undergo surface changes facilitated by specific surfactants, interacting selectively with certain biological entities or organic compounds [10].
Although magneto-fluidic systems with diverse compositions find application in technology and medicine, their macroscopic properties, microstructure, and behavioral dynamics are significantly influenced by external factors. Accurately predicting the micro- and macroscopic responses of magneto-fluidic systems, which is pivotal for developing smart materials under external influences, represents a crucial scientific challenge. Consequently, studying the interaction between the microstructure and macroscopic properties of magneto-fluidic systems becomes imperative. This involves examining how external non-invasive magnetic, mechanical, and acoustic influences collectively impact these systems, alongside exploring the dynamics of the non-magnetic inclusions within these systems when subjected to external inhomogeneous magnetic fields.
2. Nano-Disperse Magnetic Fluids: Discovery and Research Interest
Article [11] extensively details the historical synthesis process of stable colloids from magnetic nanoparticles, typically with an effective diameter of ~10 nm or larger. These colloids were initially termed magnetic fluids [11], with their dynamics termed ferrohydrodynamics [12]. Magnetic fluids represent unique artificially created materials. Magnetostatic bacteria are capable of detecting magnetic nanoparticles (MNPs) [13], yet stable liquid systems exhibiting ferromagnetic properties are absent. In the early 19th century, eminent physicists Michael Faraday and Thomas J. Seebeck studied magnetic dust under external magnetic field influences [14]. The system they investigated was unstable and settled quickly. Subsequently, Elmore measured the magnetization curves of micro-sized particles dispersed in a carrier liquid [15]. Magnetic fluids, as studied today, were first synthesized about 60 years ago in the USA [1]. They stood as pioneering artificial nano-dispersed material and became subjects of scientific research [2]. Magnetic fluids have found application in various technical devices [2,16,17], even predating the term ‘nanotechnology’ [18].
A nano-disperse magnetic fluid constitutes a colloidal system of MNPs (magnetic nanoparticles) ranging from 5 to 20 nm in diameter. These MNPs are coated with a stabilizing shell—a surfactant—dispersed within a liquid carrier. Figure 1 [19,20,21,22] provides a schematic representation of such a particle. The properties of the magnetic fluid are significantly influenced by the size of the MNPs, their concentration, and the types of surfactants used.
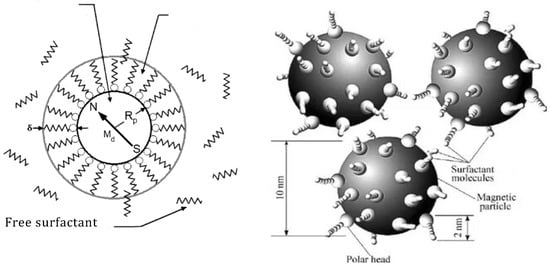
Figure 1. A schematic representation of the MNP in the magnetic fluid coated with a surfactant.
Magnetic particles within the magnetic fluid exhibit monodomain characteristics and rely on magnetic dipoles with a constant magnetic moment for description. Simultaneously, their small size prevents sedimentation at room temperature, which is facilitated by Brownian fluctuations inducing random movements of MNPs. Consequently, they uniformly disperse throughout the entire volume of the liquid carrier.
The most important characteristic of magneto-fluidic systems lies in their combination of fluidity and their capability to react (through changes in physical properties and microstructure and dynamics of the interface) to external magnetic, mechanical, and acoustic influences. The unique capabilities of magnetic fluid classify them as ‘smart’ materials and generate widespread interest among physicists, material scientists, and engineers. Ronald Rosensweig’s introduction to fundamental equations for the hydrodynamics of magnetic forces in a quasi-homogeneous magnetizable liquid medium laid the groundwork for creating a new scientific direction: ‘ferrohydrodynamics’ [2,23,24]. This field described phenomena such as thermomagnetic convection, surface instability, the levitation of a permanent magnet, and other phenomena. Subsequent experimental studies established the dependence of magnetic fluid viscosity on the external magnetic field (the magnetoviscous effect) [2] and experimentally revealed a rotational effect [12]. These experiments’ results could only be explained by considering the influence of relaxation processes. Study [2] proposes a physical model that considers the internal rotation of magnetic nanoparticles. However, this model does not account for the fact that the rotational effect occurs only in inhomogeneous magnetic fields, as demonstrated in experiment [25], and explaining this effect requires considering the structuring of magnetic fluid in an inhomogeneous magnetic field.
Engineers and scientists have shown interest in magnetic fluids. Many scientific works systematically organizing the structure, dynamics, and physical properties of magnetic fluid have been published in classical monographs [2,9] and review articles [22,26,27,28,29,30,31,32,33].
Nanotechnology has been evolving over the past 15 years, sparking renewed interest in magnetic fluids. Researchers have started viewing magnetic fluid from a different perspective. Currently, magnetic fluid is recognized as a multiphase system where magnetic nanoparticles exist as distinct elements with controlled structures and properties. Heightened interest has led to a significant surge in publications on this topic, which is reflected in Figure 2.
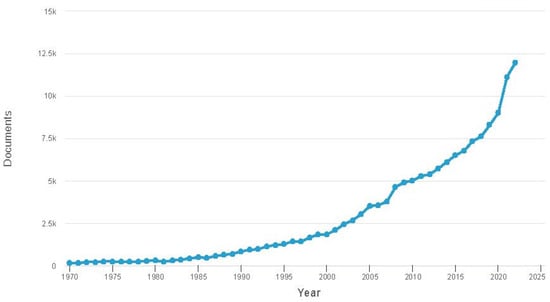
Figure 2. Dynamics of the number of publications devoted to magneto-fluidic systems, according to the Scopus database.
Magnetic fluid and magnetic nanoparticles find numerous applications, particularly in the field of biomedicine [34,35,36]. These particles serve as contrast agents in magnetic resonance imaging [37,38]. Researchers are actively investigating interactions between particles, the formation of chain aggregates and flexible clusters, and the impact of the microstructure on the macroscopic properties of magnetic fluid through both experimental and theoretical studies [39,40]. The development of magneto-fluidic systems has made it possible to significantly alter viscosity under the influence of an external magnetic field, showcasing a giant magnetoviscous effect.
The capabilities of modern chemistry enable the synthesis of various magnetic composite anisotropic MNPs achieved through extensive efforts in refining production methods that control the material, size, shape, structure, and modification of the MNP surface [41,42]. Functionalizing the MNP surface allows their utilization in creating biosensors [43], targeted drug delivery [44], magnetic separation, and the concentration of various materials [45,46], including biological objects. For instance, the antibodies of various viruses are employed in diagnostics [47], such as COVID-19 [48]. Coupled with a high-frequency magnetic field, they have the ability to generate heat and induce magnetic hyperthermia [49,50,51] for cancer treatment.
Another prevalent aspect within magneto-fluidic systems is microfluidic self-assembly [52,53]. This direction allows for the creation of distinctive nano- and microstructures when subjected to various external factors, encompassing magnetic objects [54], as well as non-magnetic objects within the magnetic fluid [55].
Presently, the exploration of magneto-fluidic systems constitutes a multidisciplinary field, encompassing condensed matter physics, magnetism, hydrodynamics, inorganic and organic chemistry, colloidal chemistry, computational and computer modeling, acoustics, engineering, and applied sciences.
3. General Information about Magnetic Fluids
Magnetic fluid is a multiphase system including solid MNPs, a surfactant stabilizer that prevents the aggregation of particles, and a carrier liquid [56], as previously mentioned. Solid fillers in the form of MNPs, typically ranging from 5 to 20 nm in size, are most frequently utilized (Figure 3) and derived from ferro- or ferrimagnetic metals and metal oxides. Figure 3 schematically depicts the dependence of a coercive force on MNP size [23,57,58]. Reducing the particle size of a magnetic material, inherently multi-domain, results in the formation of superparamagnetic single-domain particles [59,60]. This paper specifically focuses on MNPs based on an Fe3O4 magnetite. Fe3O4 magnetite possesses a reversed spinel-type structure, and its axis of light magnetization corresponds to the diagonal of the cube (third-order axis).
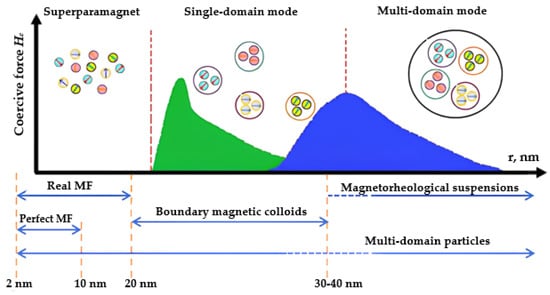
Figure 3. Influence of MNPs size on its magnetic structure and coercive force.
One can obtain superparamagnetic MNPs through physical, chemical, and biological methods. The distribution of work on the technology of magnetic fluid synthesis is depicted in Figure 4.
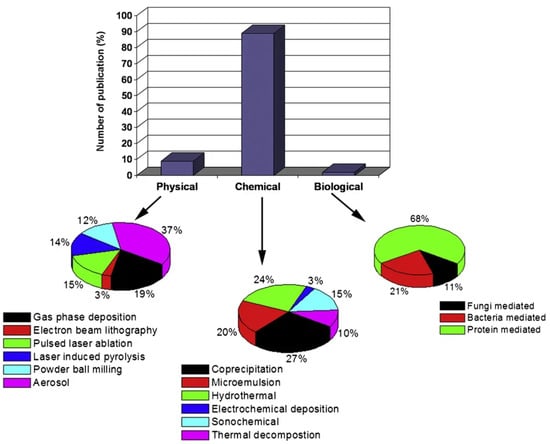
Figure 4. Publications on the technology of magnetic fluid synthesis.
Numerous works focus on chemical methods, with top-down methods being the first ones employed historically. This method involves the prolonged grinding of magnetite particles in ball mills. Subsequently, magnetite particles underwent crushing through ultrasonic treatment [63,64,65,66]. The widespread use of magnetic fluid can be attributed primarily to the advancement of chemical synthesis and the development of bottom-up technologies.
The most widespread method for synthesizing magnetic fluid is based on chemical condensation. This technology involves depositing low-frequency magnetite using a concentrated alkali solution from an aqueous solution of salts of ferrous and trivalent iron. When heated, the colloidal precipitate of MNPs is mixed with surfactants. The colloidal precipitate undergoes dissolution in the carrier liquid, a process termed peptization. The chemical condensation method is simple and technologically efficient, allowing the production of magnetic fluid based on various carrier liquids. It stands as the primary method in modern technology for magnetic fluid production [12].
The varieties of magnetic fluid distinguish themselves depending on the type of stabilization in the colloidal system. Magnetic fluid types are categorized as sterically stabilized. In this case, surfactant molecules form a solvate shell around the MNPs. Surfactant molecules physically or chemically bond with a particle. Figure 1 illustrates the model of such a particle. The surfactant molecule acts like a spring, with one end attached to the MNPs. When the particles approach, surfactant molecules contract, repelling the particles and preventing coagulation.
Another type of stabilization is ion stabilization, as proposed by Massart [67,68]. Electrostatic repulsion ensures the chemical and physical stability of the ionic magnetic fluid. The effectiveness of electrostatic repulsion strongly depends on the acidity and type of carrier fluid, which includes non-magnetic liquids such as kerosene, liquid hydrocarbons, oils, water, paraffin, organosilicon, and others. Selecting the appropriate surfactant is necessary for each type of carrier fluid. Ionic (electrostatic) stabilization is practical, mainly in the case of water-based magnetic fluids, while steric/steric + electrostatic stabilization proves useful for a wide variety of non-polar and polar carriers.
The chemical condensation method appears simple, but even a slight modification in the technological process can alter the structure of MNPs and the physical properties of the magnetic fluid. Monitoring MNPs’ structure and parameters constitutes one of the most crucial tasks. Researchers use this control to enhance the technology for producing magnetic fluid. Resolving this issue necessitates employing the following various physical methods: directly observing MNPs through atomic force microscopy, magnetic force microscopy, transmission electron microscopy, scanning electron microscopy, small-angle X-ray scattering, and magnetogranulometric analysis. Additionally, non-invasive methods, which rely on mechanical and acoustic influences and do not necessitate optical transparency of the medium, are also utilized. These noninvasive methods enable the study of dispersed magnetic fluid without replication.
This entry is adapted from the peer-reviewed paper 10.3390/nano14020222
This entry is offline, you can click here to edit this entry!
