1. Introduction
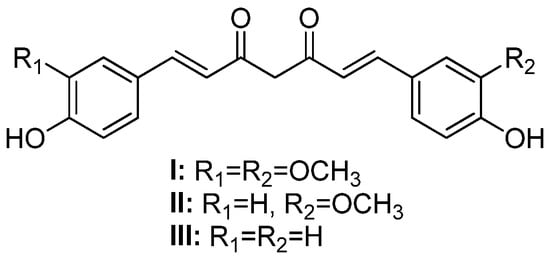
Curcumin (or 1,7-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) (
I,
Figure 1), also known as diferuloylmethane, is one of the main hydrophobic phenolic compounds found in
Curcuma longa L. (Zingiberaceae) rhizomes [
1]. Curcumin and its analogs, demethoxycurcumin (
II) and bisdemethoxycurcumin (
III), also present in
C. longa rhizomes, are commonly referred to as curcuminoids [
2].
Figure 1. Chemical structures of curcumin (I), demethoxycurcumin (II), and bisdemethoxycurcumin (III).
Besides being used as a supplement, seasoning, food preservative, flavoring, and coloring in the food industry [
3], curcumin has various biological and pharmacological activities, including antioxidant [
4], anti-inflammatory [
5], antimicrobial [
6], anticancer [
7], and antiparasitic [
8] actions. However, limitations have prevented it from being approved as a therapeutic agent. For example, curcumin is poorly bioavailable, because it is barely absorbed and rapidly metabolized [
9]. Moreover, it has low chemical stability under physiological conditions, because its β-diketone moiety is prone to hydrolysis [
10]. Furthermore, it undergoes rapid light-induced decomposition [
11]
The diverse biological activities of curcumin and its low bioavailability and stability have motivated the synthesis of curcumin analogs bearing a modified β-diketone portion or new substituents in the aromatic moiety [
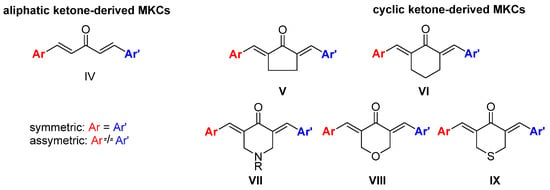
10]. Replacing the β-diketone portion with a monocarbonyl portion increases the chemical stability of the resulting monoketone curcuminoid (MKC) under physiological conditions [
12] and provides the compounds with interesting biological actions [
13]. However, MKCs (
IV,
Figure 2) have a controversial and confusing nomenclature. In the literature, the terms “diphenylpentanoids,” “dibenzylidene ketones,” “1,5-diaryl-penta-1,4-diene-3-ones,” “diarylpentanoids,” “monoketone curcuminoids,” “C5-curcuminoids,” “monocarbonyl curcuminoids,” “deketene curcumin,” and “monocarbonyl curcumin” are used to refer to them. Nevertheless, the general term “curcuminoids,” which includes the dicarbonyl compounds, has been used most often. The lack of a uniform nomenclature for MKCs not only makes searching for them in the literature challenging, but also causes their importance to be underestimated.
Figure 2. Structures of MKCs displaying an acyclic (
IV) and a cyclic C5 bridge (
V–
IX) [
14].
According to Moreira and co-workers, symmetric (Ar = Ar’) and asymmetric (Ar ≠ Ar’) MKCs are classified on the basis of the carbon atoms of the C5 unit, and the classification depends on whether these carbon atoms are part of an acyclic skeleton or belong to a cyclic fraction. When MKCs bear a cyclic C5 unit, this unit can be part of a cyclopentanone (
V), cyclohexanone (
VI), piperidin-4-one (
VII), tetrahydro-4
H-pyran-4-one (
VIII), or a tetrahydro-4
H-thiopiran-4-one (
IX) (
Figure 2) [
14].
Although extensive reviews on MKCs’ biological activities have been published over the last decade [
12,
15,
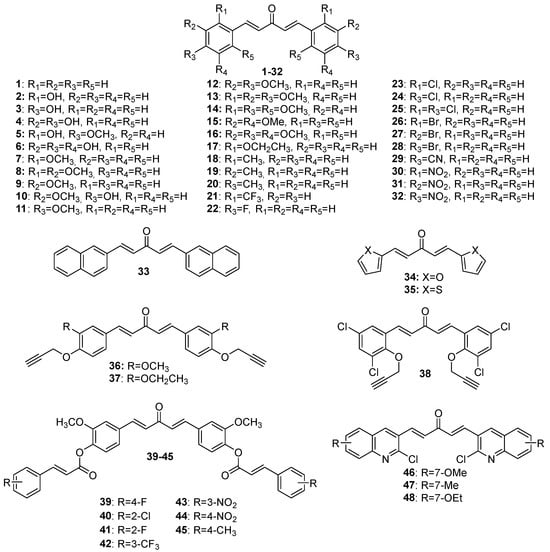
16], a review spanning a more recent period would be welcome. Here, the researchers provide an overview of the recent literature (2019–2023) on the biological activities of MKCs and address some aspects of their synthesis and biological activities (e.g., their insecticidal activity) that have not been discussed yet. The structures of MKCs
1–
48 (symmetric acetone-derived MKCs),
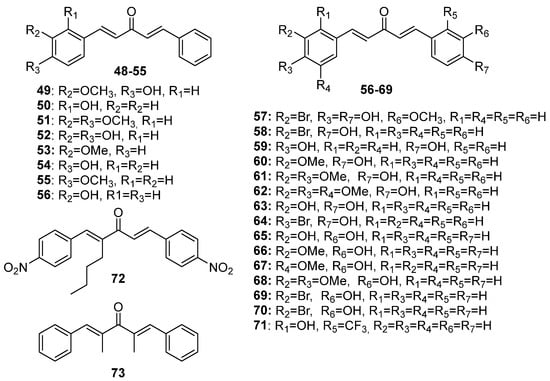
49–
73 (asymmetric acetone-derived MKCs),
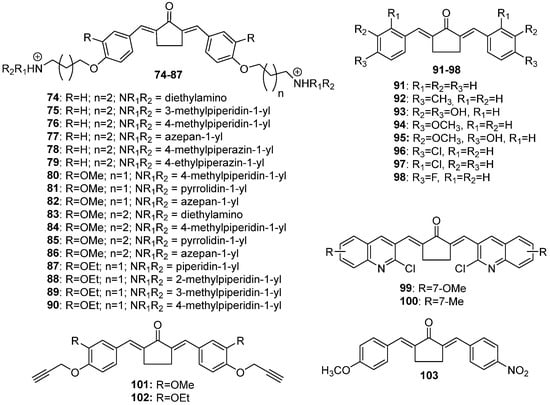
74–
103 (cyclopentanone-derived MKCs),
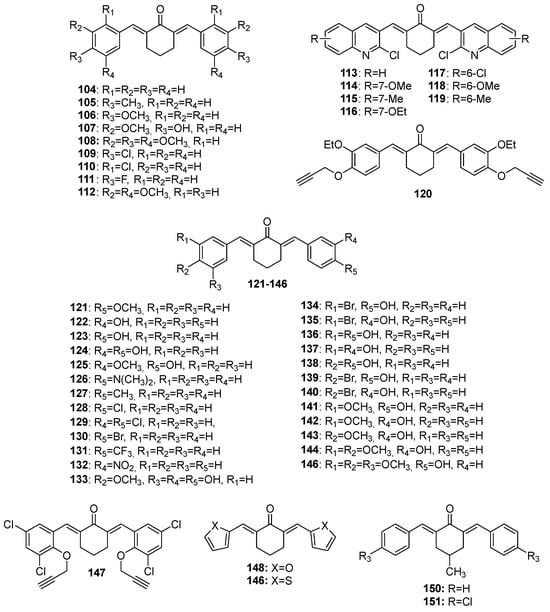
104–
151 (cyclohexanone-derived MKCs),
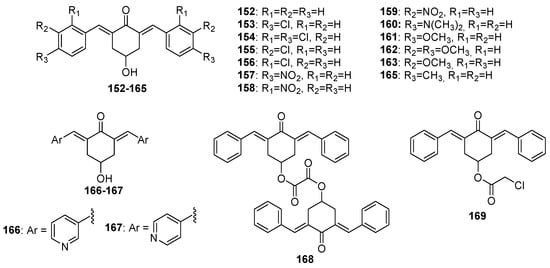
152–
169 (4-hydroxy-cyclohexanone-derived MKCs),
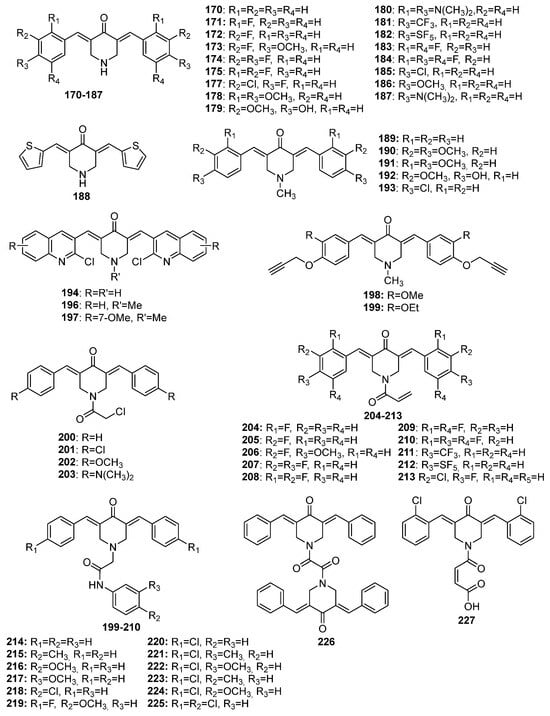
170–
227 (piperidin-4-one-derived MKCs), and
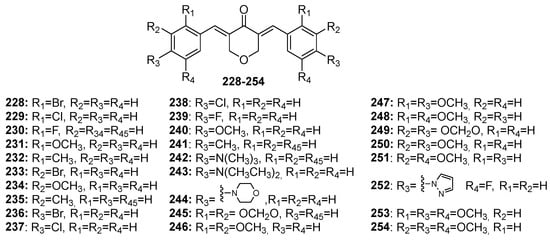
228–
254 (tetrahydro-4
H-pyran-4-one-derived MKCs) addressed in this review article are shown in
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9 respectively.
Figure 3. Chemical structures of symmetric acetone-derived MKCs 1–48.
Figure 4. Chemical structures of asymmetric acetone-derived MKCs 49–73.
Figure 5. Chemical structures of cyclopentanone-derived MKCs 74–103.
Figure 6. Chemical structures of cyclohexanone-derived MKCs 104–151.
Figure 7. Chemical structures of 4-hydroxy-cyclohexanone-derived MKCs 152–169.
Figure 8. Chemical structures of 4-piperidone-derived MKCs 170–227.
Figure 9. Chemical structures of pyran-4-one-derived MKCs 228–254.
2. Claisen–Schmidt Condensation (CSC) as a Source of MKCs
2.1. General Aspects and Stereoselectivity
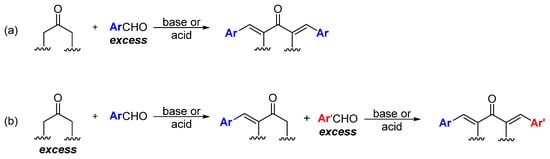
Unlike curcumin, which can be obtained from natural sources, MKCs can be only produced by using synthetic chemical methods. Although some methodologies have been proposed to synthesize MKCs [
17], Claisen–Schmidt condensation (CSC) between an aromatic aldehyde and a cyclic (e.g., cyclopentanone, cyclohexanone, piperidin-4-one, tetrahydro-4
H-pyran-4-one, or a tetrahydro-4
H-thiopiran-4-one) or aliphatic (e.g., propanone) ketone is the standard procedure to synthesize MKCs (
Scheme 1). CSC is more attractive because it is inexpensive, does not require complex experimental apparatus, and the resulting MKCs can be isolated by vacuum filtration and further purified by recrystallization. Various aromatic aldehydes can be used, because they do not undergo enolization and, hence, cannot function as the nucleophilic component of the reaction. CSC can be base- or acid-catalyzed; moreover, in both cases, excess aromatic aldehyde is employed to ensure that MKC is the final product (
Scheme 1a) [
18]. Alternatively, excess ketone, instead of excess aromatic aldehyde, can be used, so that the ketone condenses with one aldehyde only. The resulting product can then react with a different aromatic aldehyde to produce an asymmetric MKC (
Scheme 1b) [
19]. Recently, Yadav and Wagh published an extensive review of CSC [
20], therefore, the researchers will only address some selected aspects of this reaction.
Scheme 1. Synthesis of symmetric (
a) and asymmetric (
b) MKC by Claisen–Schmidt condensation [
19].
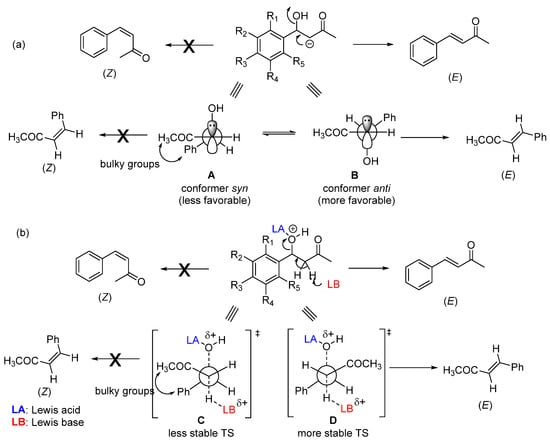
Enone formation from the aromatic aldol is thermodynamically favored, driven by the extended conjugation between the carbonyl and the aromatic ring. A
trans-double bond (
E configuration) is preferentially formed in an acid- or base-catalyzed CSC (
Scheme 2). This stereoselectivity arises during the elimination step and stems from both steric and stereoelectronic factors. In the case of a base-catalyzed CSC, the hydroxyl group is expelled as a hydroxide anion (OH
−) through an E1cb mechanism that requires the carbanion (enolate ion)-filled p orbital to be in
anti with the C–OH bond σ* orbital [
21,
22]. Among the two possible conformers (A and B,
Scheme 2a) that allow these orbitals to be in an anti-arrangement, conformer B is the most stable—the bulkiest phenyl and acetyl groups are located on opposite sides. Consequently, conformer B reacts faster (through a lower-energy transition state) than conformer A to produce the double bond with
E configuration. As for the acid-catalyzed CSC (
Scheme 2b), the stereoselectivity originates from an E1-like transition state, where the C–O bond cleavage is considerable.
Scheme 2. Possible conformers involved in double-bond formation through the E1cb mechanism (base-catalyzed CSC) (a) and E1-like mechanism (acid-catalyzed CSC) (b).
2.2. Base-Catalyzed Claisen–Schmidt Condensation
In the standard CSC procedure, acetone, cyclopentanone, cyclohexanone, piperidin-4-one, tetrahydro-4
H-pyran-4-one, or tetrahydro-4
H-thiopiran-4-one reacts with an aromatic aldehyde in a sodium hydroxide (NaOH) alcoholic (methanolic or ethanolic) solution. Initially, an aqueous NaOH solution is added to ethanol (EtOH), and the resulting solution is then added to the reaction vessel containing the ketone at 0 °C and stirred for a few minutes. Next, the aromatic aldehyde is added to the reaction vessel and stirred at room temperature. In some methodologies, at the end of the reaction, the reaction mixture is neutralized by adding a hydrochloric acid (HCl) solution to the vessel [
23]. In most cases, the resulting solid is separated from the reaction mixture by vacuum filtration and washed with ice water to remove the excess base. Finally, the solid product is dried and recrystallized from hexane/ethyl acetate or EtOH to obtain pure crystals [
18,
23,
24,
25,
26].
Homogeneous basic catalysts allow the CSC reaction to be conducted at lower temperatures in a shorter time. Protocols based on catalysts like NaOH/EtOH [
18,
23,
27] and NaOH/MeOH [
24,
28,
29,
30] have been reported. Nevertheless, these methods have certain limitations, such as the following: excess or stoichiometric amounts of reactants are employed, and the reactants can be corrosive and may not be recoverable.
Besides NaOH, calcium hydroxide (Ca(OH)
2) has been used as a basic catalyst in CSC to obtain MKCs. Zhang and co-workers synthesized a series of MKCs by CSC between (
E)-4-phenylbut-3-en-2-one and benzaldehyde catalyzed by Ca(OH)
2 in a diluted EtOH medium (20%
v/
v) at 60 °C. A reaction for 48 h produced dibenzylideneacetone
1 (
Figure 3) in a 47% yield. Then, the authors tested different experimental conditions of temperature, catalyst amount, and reaction scale. A temperature of 80 °C, Ca(OH)
2 at 10%, and 10 or 100 mmol of each reagent produced compound
1 in 81% and 80% yield, respectively, after column chromatography and filtration. After the authors optimized the reaction conditions, they tested other aromatic aldehydes and demonstrated that electron-deficient aldehydes are preferable because they produce a higher MKC yield after the product is purified [
31].
2.3. Acid-Catalyzed CSC
Although some experimental aspects have led base-catalyzed CSC to be more often used to synthesize MKCs, acid-catalyzed CSC is preferred when the aromatic aldehyde structure contains acid sites other than α-carbonyl hydrogen (e.g., the hydrogen of phenol hydroxyl groups). Acetic acid/HCl gas [
32], acetic acid/H
2SO
4 [
33], or Lewis acids like niobium pentachloride [
34] have recently been employed to obtain MKCs.
3. Synthesis of MKCs through Oxidative Catalysis
Waldron and co-workers designed a method to synthesize dibenzylidene acetone (
1) and (1
E,4
E)-1,5-
bis(4-methoxyphenyl)penta-1,4-dien-3-one (
11) from benzyl alcohol and 4-methoxybenzyl alcohol in a flow system composed of three micropacked bed reactors operating at 115, 130, and 120 °C, respectively. The methodology employs gold–palladium (Au–Pd) nanoparticles supported on titanium dioxide (TiO
2) and produces MKCs as secondary products. In this system, the compounds are synthesized via oxidation, aldolic condensation, and reduction pathways; moreover, AuPd/TiO
2, TiO
2 anastase, and 1 wt% Pt/TiO
2 are used for oxidation, C-C coupling, and reduction, respectively. During the coupling between the alcohol and the ketone, two aldehyde molecules couple to the ketone to produce MKCs
1 and
11 (
Figure 3). Lower temperatures (80–100 °C) promote selectivity for the desired product (>80%), while higher temperatures favor benzaldehyde production for the coupling reaction between benzaldehyde and the ketone [
35].
4. Using Ionic Liquids and Apolar Solvents in the Synthesis of MKCs
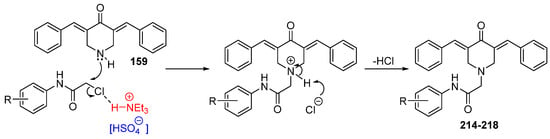
Interest in using ionic liquids (ILs) in aldol condensation reactions has increased because ILs are a safer and cleaner reaction medium than organic solvents [
21]. Thus, some authors have used ILs to obtain MKCs. For instance, Subhedar and co-workers designed a method to synthesize compounds
174–
185 (
Figure 8) by using the IL [Et
3NH
+][HSO
4−] as a medium/catalyst via a one-pot multicomponent approach. The authors proposed that the reaction starts with aldehyde carbonyl protonation by [Et
3NH
+][HSO
4−], which is followed by piperidone enolization and further nucleophilic attack of the carbonyl of the aromatic aldehyde, with the consequent formation of a C–C bond. Subsequent protonation and water elimination generates compound
160. The IL increases the electrophilicity of the carbon atom of the C–Cl bond in 2-chloro-
N-phenylacetamide and accelerates C–N bond formation through the nucleophilic substitution of intermediate
159 to produce compounds
214–
218 (
Figure 8), as shown in
Scheme 3 [
21].
Scheme 3. Mechanism of the IL-assisted formation of MKCs
214–
218 from
159 [
21].
This entry is adapted from the peer-reviewed paper 10.3390/futurepharmacol4010006