Corneal stromal stem cells (CSSCs) are of particular interest in regenerative ophthalmology, offering a new therapeutic target for corneal injuries and diseases. CSSC-derived exosomes exhibit significant potential for modulating inflammation, promoting tissue repair, and addressing corneal transparency. Additionally, the rejuvenation potential of CSSCs through epigenetic reprogramming adds to the evolving regenerative landscape. The imperative for clinical trials and human studies to seamlessly integrate these strategies into practice is emphasized. This points towards a future where CSSC-based therapies, particularly leveraging exosomes, play a central role in diversifying ophthalmic regenerative medicine.
1. Introduction
Impairment of corneal transparency and compromised refractive function are prominent causes of blindness. It is estimated that between 4.9 and 5.5 million people worldwide are bilaterally blind or have bilateral visual impairment, both resulting from corneal opacification [
1,
2,
3,
4]. It is estimated that an additional 6.2 million people are unilaterally blind. According to the WHO, 1.9 million instances of this opacification of the cornea are due to trachoma, an infection caused by the bacteria Chlamydia trachomatis [
5,
6]. The remaining cases of corneal opacification are due to a great number of other factors, such as onchocerciasis, vitamin A deficiency, tumors, and wounds [
7,
8].
The use of stem cells and stem cell stimulation to restore cornea clarity presents a potential strategy to address the shortage of donor corneas for transplantation by making transplantations a lower priority. In the case of limbal epithelial cells, stem cell transplantation is already a clinical routine [
19,
20,
21,
22]. Utilizing the regenerative potential of a patient’s own stem cells eliminates the dependency on external donor sources, mitigating the challenges associated with donor cornea scarcity. Autologous stem cell therapies involve isolating and cultivating a patient’s own stem cells, typically from the limbus or other corneal tissues, and then reintroducing them to the damaged cornea.
2. Corneal Anatomy
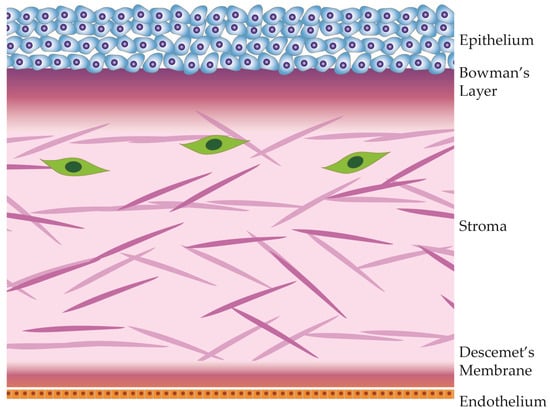
The cornea, a transparent, avascular tissue forming the anterior part of the eye, protects the contents of the eye and provides the main ocular refractive power. Comprising five distinct layers, which are illustrated in
Figure 1, the cornea exhibits a meticulously organized structure, with each layer contributing to its unique optical and biomechanical properties [
33,
34].
Figure 1. Anatomical diagram of the cornea and its 5 distinct layers.
The corneal epithelium, the outermost layer of the cornea, is a highly specialized and transparent tissue made of layers of tightly packed epithelial cells. This layer is the first protective barrier against environmental factors, including pathogens and foreign particles. The corneal epithelium is also the first layer to refract light, contributing to visual acuity [
34,
35]. This stratified epithelium undergoes continuous renewal, with limbal epithelial stem cells dividing asymmetrically and migrating along the basal layer to then divide further and migrate toward the surface.
Beneath the epithelium lies Bowman’s layer, an acellular, collagenous sheet providing structural support to the cornea. Though relatively thin, it contributes to the cornea’s tensile strength, provides a basement layer to the epithelium, and acts as a barrier against epithelial ingrowth [
43]. This layer also houses the sub-basal plexus, which sends nerve fiber branches orthogonally into the anterior epithelium [
44,
45].
Collagen fibrils within the human cornea are organized, forming bundles known as lamellae that intersect across the cornea’s width [
48]. In the anterior region, these lamellae exhibit more pronounced interweaving, contributing significantly to the cornea’s shape and transparency, particularly relevant to its refractive properties. The alignment of collagen fibrils also contributes to corneal transparency, creating a pseudo-crystalline structure that facilitates the transmission of light through the cornea [
46,
47]. The distribution and organization of glycosaminoglycans in the corneal stroma exhibit a gradient along the anterior–posterior axis, resulting in differential responses to stress and swelling in various parts of the corneal stroma [
49,
50].
Situated beneath the stroma, Descemet’s membrane is a basement membrane that provides additional structural support. This acellular layer plays a role in maintaining corneal shape and integrity. It also serves as a substrate for endothelial cell attachment [
52].
The innermost layer, the corneal endothelium, is a monolayer of cells that regulates corneal dehydration. Through active ion transport, the endothelium ensures that the stroma remains dehydrated, preventing corneal swelling. Any swelling of the cornea would result in deformation and loss of visual acuity. Unlike other corneal layers, the endothelium has limited regenerative capacity, making its health vital for overall corneal function. Dysfunction of the corneal endothelium is the most common cause of corneal transplantation in industrialized countries [
53].
3. Identity of Corneal Stromal Stem Cells
3.1. Origins and Potency
The mammalian eye undergoes construction in developmental stages originating from the neural crest, which gives rise to three distinct sources of precursor eye cells: the neural ectoderm, surface ectoderm, and periocular mesenchyme [
54,
55]. Specifically, the periocular mesenchyme contributes to the formation of stromal cells responsible for building the stroma, eventually transforming into keratocytes [
56]. Within the periocular mesenchyme, these cells play a vital role in the initial construction and remodeling of the extracellular matrix (ECM) into the stroma, characterized by a unique structure and deposited proteins ensuring transparency [
57]. As the tissue develops, apoptosis occurs, leaving behind a small population that expresses crucial proteins for corneal stromal cell function, including crystallins [
58]. Tracking the differentiation from neural crest cells to quiescent keratocytes involves observing the disappearance of PAX6, a marker of early eye development, and the emergence of CD34, a general marker of mesenchymal stem cells [
59].
3.2. Molecular Markers
Stromal stem cells in the cornea are characterized by their unique molecular markers and location within the tissue. Corneal keratocytes, residing within the corneal stroma, exhibit distinctive molecular markers that are integral to their identification and functional characterization. Among these markers, Pax6 and ABCG2 serve as key indicators of keratocyte progenitor cells, highlighting their quiescent and stem cell-like properties, respectively. Other general mesenchymal stem cell markers, such as MSC markers such as CD73, CD90, CD105, and CD140b/PDGFRβ, are also expressed [
61]. ABCB5, a member of the ATP-binding cassette transporter family, is another noteworthy molecular marker associated with corneal keratocytes, emphasizing their stem cell characteristics [
62,
63]. Pax6, ABCG2, and ABCB5 are also present in corneal epithelial cells, particularly the corneal epithelial stem cells [
59,
63]. It is possible to distinguish between stromal and epithelial stem cells as the two are separated by Bowman’s layer and the stromal cells express mesenchymal markers that are not found in epithelial cells [
61].
These molecular markers play a pivotal role in distinguishing keratocytes from other corneal cell types, providing valuable insights into their regenerative potential. Understanding the nuanced molecular profile of corneal keratocytes is essential for advancing research on corneal biology, tissue engineering, and therapeutic interventions aimed at enhancing corneal health and regeneration.
Corneal stromal stem cells are distinct cell populations with unique characteristics. According to the in vitro behavior of CSSCs, they can be characterized as MSCs according to the guidelines set by the International Society of Cellular Therapy [
65]. While both CSSC and MSC have the potential to differentiate into various cell types, there are notable differences between them. They each exhibit specific lineage commitments. Corneal stromal stem cells have the primary capacity to differentiate into corneal keratocytes, which are responsible for the maintenance of the corneal stroma. In contrast, mesenchymal stem cells are a more broadly distributed population found in various tissues, such as bone marrow and adipose tissue. Both of these cells possess in vitro trilineage differentiation potential, meaning they can differentiate into cells from three different lineages: osteogenic, chondrogenic, and adipogenic lineages. This broader differentiation capacity makes MSCs versatile for regenerative applications in various tissues and systems, whereas corneal stromal stem cells are more specialized in their role, specifically contributing to corneal health and transparency.
3.3. Location
Stromal stem cells are primarily situated in the anterior stroma, close to the boundary with the corneal epithelium [
69,
70,
71]. Their strategic positioning allows them to respond promptly to injuries and insults to the cornea. Within the limbal stroma, a niche enriched with extracellular matrix components and signaling molecules, there is a greater number of corneal stromal stem cells that maintain a quiescent state and regenerative potential. These cells serve as a reservoir of progenitor cells that can differentiate into functional keratocytes, the specialized cells responsible for preserving the transparent corneal stroma. Their strategic positioning at the limbus not only allows for rapid response to corneal injuries or insults but also plays a role in preserving the cornea’s vascular privilege. The stromal cells maintain tissue homeostasis through macrophage modulation [
72].
3.4. Homeostasis and Tissue Integrity
Even in the absence of injury, stromal stem cells help maintain corneal homeostasis by monitoring the health of keratocytes and participating in the renewal of the stromal matrix. This continual surveillance ensures the cornea’s structural and functional integrity. Stromal stem cells play a key role in ensuring corneal transparency and structural integrity by participating in a bidirectional crosstalk with the corneal epithelium, which helps regulate the renewal of epithelial and stromal cells.
3.5. Regeneration of Keratocytes
When the cornea is injured or experiences cellular turnover, stromal stem cells are activated to proliferate and differentiate into keratocytes. Keratocytes are the specialized cells responsible for maintaining the extracellular matrix of the corneal stroma, which is primarily composed of collagen fibrils. This regenerative process is essential for restoring the structural integrity and transparency of the cornea.
3.6. Maintenance of Corneal Clarity
Stromal stem cells continuously contribute to corneal clarity by replenishing damaged or aged keratocytes. This ongoing turnover ensures that the cornea remains transparent and free from opacities, which could compromise visual acuity. Corneal keratocytes are responsible for producing and organizing the extracellular matrix, a critical component of corneal transparency. It should be noted that there is a decrease in epithelial stem cell proliferative capacity as patients age. Telomere length and marker expression remain unchanged, at least in corneal epithelial stem cells [
78].
4. Interactions within the Stromal Stem Cell Niche
The most obvious function of the epithelium with regards to the stroma is as a barrier. The epithelium is the first line any exterior threat, be it viral, bacterial, or physical, must traverse before entering the stroma.
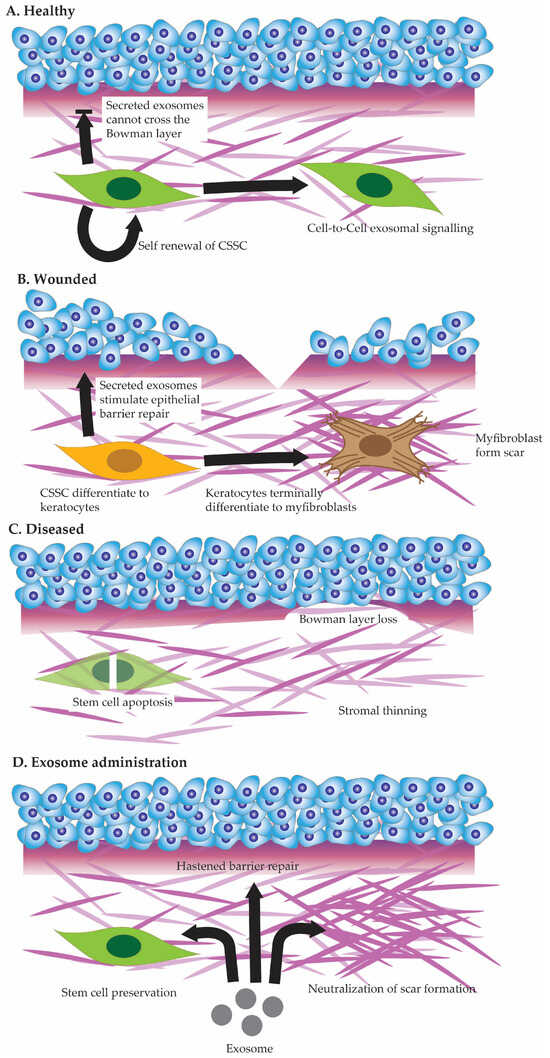
Corneal stromal stem cells interact with each other within the stromal matrix (see
Figure 2A), arranging a dynamic balance that contributes to tissue homeostasis and repair [
90,
91]. Cell-to-cell communication among stromal stem cells involves various signaling pathways and molecular cues, influencing their quiescence, proliferation, and differentiation [
92].
Figure 2. Cell state in healthy cornea (A), injured cornea (B), and diseased cornea (C). Potential future benefits of exosome administration to corneal healing (D).
The interaction between genes and stromal–epithelial crosstalk is crucial for maintaining corneal homeostasis. Key genes, including Krüppel-like factor-4 (Klf4) [
97], Pax6, and the Inhibitor of differentiation (Id), are pivotal in regulating the dynamic communication between the corneal stroma and epithelium.
Klf4, a highly expressed transcription factor in the cornea, plays a central role in the stromal–epithelial crosstalk [
98]. It regulates epithelial integrity and permeability, ensuring effective communication between corneal layers. Experiments in transgenic mice with reduced Klf4 expression resulted in increased epithelial cell layers and a disrupted barrier function, highlighting Klf4’s importance in maintaining the equilibrium between the stroma and epithelium [
99].
The Pax6 gene, known for its involvement in eye development, also influences stromal–epithelial crosstalk [
104,
105]. Changes in Pax6 levels in transgenic mice led to alterations in the morphology of epithelial, stromal, and endothelial cells, affecting cell adhesion and hydration [
106]. This demonstrates how Pax6 variations disrupt the interplay between corneal layers. In humans, mutations in the Pax6 gene are linked to aniridia and aniridia-related keratopathies [
107,
108,
109].
In addition to Klf4 and Pax6, the Inhibitor of differentiation (Id) genes contribute to stromal–epithelial crosstalk [
110]. Experiments in vitro found that Id genes regulate cell proliferation and differentiation and are differentially expressed in corneal fibroblasts compared to myofibroblasts [
111].
In the event of a more extensive injury that damages both the corneal epithelium and the underlying stroma (See
Figure 2B), the interactions between corneal stromal stem cells and epithelial cells become even more complex [
116,
117,
118]. Stromal stem cells play a multifaceted role by not only participating in the regeneration of the epithelium but also contributing to stromal repair [
119].
5. Stromal Stem Cell Defects
Corneal stromal stem cell defects constitute a significant area of concern, impacting the regenerative capacity and maintenance of corneal health. These defects may arise from various factors, including genetic mutations or environmental influences, leading to a compromised ability of stromal stem cells to proliferate and differentiate effectively. The precise role stromal stem cell defects play in various corneal stromal diseases (See
Figure 2C)—be it hereditary or acquired—remains to be studied [
120].
Understanding the molecular basis of corneal stromal stem cell defects is paramount for developing targeted therapeutic interventions. It involves deciphering the intricate signaling pathways and genetic factors that regulate the behavior of these stem cells. Defects in signaling molecules or disruptions in the microenvironment surrounding stromal stem cells can hinder their normal function. Emerging technologies, such as gene editing and stem cell therapies, offer promising avenues for addressing these defects, with the potential to enhance the regenerative capabilities of corneal stromal stem cells.
Stromal stem cell defects in the cornea can instigate a cascade of events leading to opacification and vascularization, profoundly affecting visual clarity and corneal health. The corneal stromal stem cell is unique among the other stem cells in the cornea as it must self-renew, direct anti-fibrotic wound healing, and immunomodulate [
121,
122]. The corneal stromal stem cells also differ from their descendent cells, the corneal fibroblasts. Both are capable of producing collagen-fibrillar ECM.
6. Stromal Stem Cell Therapy—Potential for Exosome Therapy
Exosome therapy has emerged as a promising avenue for harnessing the therapeutic potential of corneal stromal stem cells. Both by replicating corneal stromal stem cell secretions to target epithelial cells or by affecting CSSC directly [
132,
133]. Exosomes are a type of extracellular vesicles released by various cell types, including stromal stem cells, that carry a cargo of bioactive molecules, such as proteins, lipids, and nucleic acids [
134]. When applied to corneal stromal stem cells, exosomes can act as messengers, facilitating intercellular communication and inducing certain cellular behaviors [
135]. Studies have shown that exosomes derived from stromal stem cells can enhance the regenerative properties of recipient cells by promoting cell proliferation, modulating inflammation, and regulating extracellular matrix remodeling (See
Figure 2D) [
133].
Furthermore, exosome therapy for corneal stromal stem cells aligns with the paradigm of regenerative medicine, offering a more targeted and precise approach to address specific cellular deficiencies. By delivering bioactive factors directly to the affected cells, exosomes can modulate the molecular and cellular environment, fostering a conducive milieu for enhanced tissue regeneration. This therapeutic strategy holds particular promise in treating conditions where corneal stromal stem cell defects lead to opacification and vascularization, as exosomes can potentially mediate anti-angiogenic effects and promote the restoration of corneal transparency.
7. Stromal Stem Cell Rejuvenation
Corneal stromal stem cell rejuvenation is a novel approach in regenerative medicine. With millions of people worldwide awaiting corneal transplantation and the significant challenges associated with donor cornea scarcity, strategies focusing on the rejuvenation of corneal stromal stem cells offer a potential future paradigm shift: Regeneration instead of Transplantation. The process would involve stimulating the proliferation and differentiation of existing stromal stem cells within the cornea, potentially replenishing the regenerative pool and enhancing the tissue’s inherent capacity for repair. Various techniques, including growth factors, gene therapy, and tissue engineering, are being explored to invigorate corneal stromal stem cells, promoting their functionality and augmenting the overall regenerative potential of the cornea. Epigenetic reprogramming can rejuvenate the stem cell population. The activation of the Yamanaka factors (OCT4, SOX2, KLF4, and MYC) [
144] within differentiated cells leads to the induction of pluripotency, giving rise to induced pluripotent stem cells [
145]. These stem cells could be used to replenish depleted stromal stem cell niches in patients. A
Beyond these methods to rejuvenate stem cell populations, there are other, even less direct approaches. These involve complex, systemic interactions such as diet and circadian rhythm. Such approaches are studied, particularly with regards to more general age-related stem cell depletions [
149].
By rejuvenating corneal stromal stem cells, researchers and clinicians aim to not only alleviate the burden of corneal blindness—especially relevant in lower-income countries-but also circumvent the challenges associated with traditional corneal transplantation. The scarcity of donor corneas, particularly in regions with limited infrastructure for tissue donation and eye banking, poses a substantial barrier to addressing the global demand for corneal grafts. Rejuvenating endogenous stromal stem cells offers a self-sustaining and potentially more scalable solution, reducing dependence on external donor sources. This innovative approach, if successful, could revolutionize corneal care, providing a viable alternative for patients in need of corneal tissue replacement and contributing to a more equitable and accessible solution for the treatment of corneal disorders on a global scale.
This entry is adapted from the peer-reviewed paper 10.3390/cells13020163