The transient receptor potential vanilloid 4 (TRPV4) specifically functions as a mechanosensitive ion channel and is responsible for conveying changes in physical stimuli such as mechanical stress, osmotic pressure, and temperature. TRPV4 enables the entry of cation ions, particularly calcium ions, into the cell. Activation of TRPV4 channels initiates calcium oscillations, which trigger intracellular signaling pathways involved in a plethora of cellular processes, including tissue repair. Widely expressed throughout the body, TRPV4 can be activated by a wide array of physicochemical stimuli, thus contributing to sensory and physiological functions in multiple organs.
- TRPV4
- calcium oscillations
- tissue repair
- fibrosis
1. Introduction
2. Function of TRPV4-Evoked Ca2+ Oscillations in Injury Repair and Fibrosis
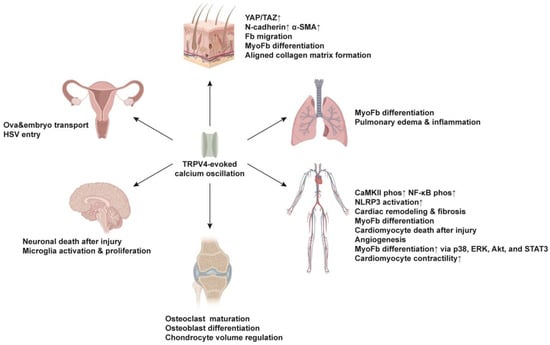
2.1. Skin
2.2. Lung
2.3. Cardiovascular System
2.4. Skeletal System
2.5. Nervous System
2.6. Reproductive System
This entry is adapted from the peer-reviewed paper 10.3390/ijms25021179
References
- White, J.P.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016, 96, 911–973.
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417.
- Moran, M.M. TRP Channels as Potential Drug Targets. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 309–330.
- Cosens, D.J.; Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969, 224, 285–287.
- Montell, C. The TRP superfamily of cation channels. Sci. STKE 2005, 2005, re3.
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261.
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. Biol. Sci. 2020, 287, 20201309.
- Rosenbaum, T.; Islas, L.D. Molecular Physiology of TRPV Channels: Controversies and Future Challenges. Annu. Rev. Physiol. 2023, 85, 293–316.
- Ho, C.Y.; Gu, Q.; Lin, Y.S.; Lee, L.Y. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir. Physiol. 2001, 127, 113–124.
- Bohlen, C.J.; Priel, A.; Zhou, S.; King, D.; Siemens, J.; Julius, D. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 2010, 141, 834–845.
- Shibasaki, K.; Murayama, N.; Ono, K.; Ishizaki, Y.; Tominaga, M. TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J. Neurosci. 2010, 30, 4601–4612.
- Nilius, B.; Biro, T.; Owsianik, G. TRPV3: Time to decipher a poorly understood family member! J. Physiol. 2014, 592, 295–304.
- Chaigne, S.; Barbeau, S.; Ducret, T.; Guinamard, R.; Benoist, D. Pathophysiological Roles of the TRPV4 Channel in the Heart. Cells 2023, 12, 1654.
- Sharma, S.; Goswami, R.; Zhang, D.X.; Rahaman, S.O. TRPV4 regulates matrix stiffness and TGFβ1-induced epithelial-mesenchymal transition. J. Cell Mol. Med. 2019, 23, 761–774.
- Jiang, D.; Christ, S.; Correa-Gallegos, D.; Ramesh, P.; Kalgudde Gopal, S.; Wannemacher, J.; Mayr, C.H.; Lupperger, V.; Yu, Q.; Ye, H.; et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 2020, 11, 5653.
- Wan, L.; Jiang, D.; Correa-Gallegos, D.; Ramesh, P.; Zhao, J.; Ye, H.; Zhu, S.; Wannemacher, J.; Volz, T.; Rinkevich, Y. Connexin43 gap junction drives fascia mobilization and repair of deep skin wounds. Matrix Biol. 2021, 97, 58–71.
- Rajendran, V.; Ramesh, P.; Dai, R.; Kalgudde Gopal, S.; Ye, H.; Machens, H.G.; Adler, H.; Jiang, D.; Rinkevich, Y. Therapeutic Silencing of p120 in Fascia Fibroblasts Ameliorates Tissue Repair. J. Investig. Dermatol. 2023, 143, 854–863.e4.
- Che, H.; Yue, J.; Tse, H.F.; Li, G.R. Functional TRPV and TRPM channels in human preadipocytes. Pflug. Arch. 2014, 466, 947–959.
- Gilchrist, C.L.; Leddy, H.A.; Kaye, L.; Case, N.D.; Rothenberg, K.E.; Little, D.; Liedtke, W.; Hoffman, B.D.; Guilak, F. TRPV4-mediated calcium signaling in mesenchymal stem cells regulates aligned collagen matrix formation and vinculin tension. Proc. Natl. Acad. Sci. USA 2019, 116, 1992–1997.
- Szabó, I.L.; Herczeg-Lisztes, E.; Szegedi, A.; Nemes, B.; Paus, R.; Bíró, T.; Szöllősi, A.G. TRPV4 Is Expressed in Human Hair Follicles and Inhibits Hair Growth In Vitro. J. Investig. Dermatol. 2019, 139, 1385–1388.
- Rahman, M.; Mukherjee, S.; Sheng, W.; Nilius, B.; Janssen, L.J. Electrophysiological characterization of voltage-dependent calcium currents and TRPV4 currents in human pulmonary fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L603–L614.
- Mukherjee, S.; Kolb, M.R.; Duan, F.; Janssen, L.J. Transforming growth factor-β evokes Ca2+ waves and enhances gene expression in human pulmonary fibroblasts. Am. J. Respir. Cell Mol. Biol. 2012, 46, 757–764.
- Janssen, L.J.; Farkas, L.; Rahman, T.; Kolb, M.R. ATP stimulates Ca2+-waves and gene expression in cultured human pulmonary fibroblasts. Int. J. Biochem. Cell Biol. 2009, 41, 2477–2484.
- Rahaman, S.O.; Grove, L.M.; Paruchuri, S.; Southern, B.D.; Abraham, S.; Niese, K.A.; Scheraga, R.G.; Ghosh, S.; Thodeti, C.K.; Zhang, D.X.; et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J. Clin. Investig. 2014, 124, 5225–5238.
- Adapala, R.K.; Katari, V.; Teegala, L.R.; Thodeti, S.; Paruchuri, S.; Thodeti, C.K. TRPV4 Mechanotransduction in Fibrosis. Cells 2021, 10, 3053.
- Haywood, N.; Ta, H.Q.; Zhang, A.; Charles, E.J.; Rotar, E.; Noona, S.T.; Salmon, M.; Daneva, Z.; Sonkusare, S.K.; Laubach, V.E. Endothelial Transient Receptor Potential Vanilloid 4 Channels Mediate Lung Ischemia-Reperfusion Injury. Ann. Thorac. Surg. 2022, 113, 1256–1264.
- Ottolini, M.; Hong, K.; Cope, E.L.; Daneva, Z.; DeLalio, L.J.; Sokolowski, J.D.; Marziano, C.; Nguyen, N.Y.; Altschmied, J.; Haendeler, J.; et al. Local Peroxynitrite Impairs Endothelial Transient Receptor Potential Vanilloid 4 Channels and Elevates Blood Pressure in Obesity. Circulation 2020, 141, 1318–1333.
- Toumpanakis, D.; Chatzianastasiou, A.; Vassilakopoulou, V.; Mizi, E.; Dettoraki, M.; Perlikos, F.; Giatra, G.; Mikos, N.; Theocharis, S.; Vassilakopoulos, T. TRPV4 Inhibition Exerts Protective Effects Against Resistive Breathing Induced Lung Injury. Int. J. Chron. Obs. Pulmon Dis. 2022, 17, 343–353.
- Adapala, R.K.; Kanugula, A.K.; Paruchuri, S.; Chilian, W.M.; Thodeti, C.K. TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation. Basic. Res. Cardiol. 2020, 115, 14.
- Ahn, M.S.; Eom, Y.W.; Oh, J.E.; Cha, S.K.; Park, K.S.; Son, J.W.; Lee, J.W.; Youn, Y.J.; Ahn, S.G.; Kim, J.Y.; et al. Transient receptor potential channel TRPV4 mediates TGF-β1-induced differentiation of human ventricular fibroblasts. Cardiol. J. 2020, 27, 162–170.
- Hatano, N.; Itoh, Y.; Muraki, K. Cardiac fibroblasts have functional TRPV4 activated by 4alpha-phorbol 12,13-didecanoate. Life Sci. 2009, 85, 808–814.
- Liao, J.; Wu, Q.; Qian, C.; Zhao, N.; Zhao, Z.; Lu, K.; Zhang, S.; Dong, Q.; Chen, L.; Li, Q.; et al. TRPV4 blockade suppresses atrial fibrillation in sterile pericarditis rats. JCI Insight 2020, 5, e137528.
- Adapala, R.K.; Thoppil, R.J.; Luther, D.J.; Paruchuri, S.; Meszaros, J.G.; Chilian, W.M.; Thodeti, C.K. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J. Mol. Cell Cardiol. 2013, 54, 45–52.
- Peana, D.; Polo-Parada, L.; Domeier, T.L. Arrhythmogenesis in the aged heart following ischaemia-reperfusion: Role of transient receptor potential vanilloid 4. Cardiovasc. Res. 2022, 118, 1126–1137.
- Veteto, A.B.; Peana, D.; Lambert, M.D.; McDonald, K.S.; Domeier, T.L. Transient receptor potential vanilloid-4 contributes to stretch-induced hypercontractility and time-dependent dysfunction in the aged heart. Cardiovasc. Res. 2020, 116, 1887–1896.
- Jones, J.L.; Peana, D.; Veteto, A.B.; Lambert, M.D.; Nourian, Z.; Karasseva, N.G.; Hill, M.A.; Lindman, B.R.; Baines, C.P.; Krenz, M.; et al. TRPV4 increases cardiomyocyte calcium cycling and contractility yet contributes to damage in the aged heart following hypoosmotic stress. Cardiovasc. Res. 2019, 115, 46–56.
- Che, H.; Xiao, G.S.; Sun, H.Y.; Wang, Y.; Li, G.R. Functional TRPV2 and TRPV4 channels in human cardiac c-kit(+) progenitor cells. J. Cell Mol. Med. 2016, 20, 1118–1127.
- Balducci, V.; Faris, P.; Balbi, C.; Costa, A.; Negri, S.; Rosti, V.; Bollini, S.; Moccia, F. The human amniotic fluid stem cell secretome triggers intracellular Ca2+ oscillations, NF-κB nuclear translocation and tube formation in human endothelial colony-forming cells. J. Cell Mol. Med. 2021, 25, 8074–8086.
- Zou, Y.; Zhang, M.; Wu, Q.; Zhao, N.; Chen, M.; Yang, C.; Du, Y.; Han, B. Activation of transient receptor potential vanilloid 4 is involved in pressure overload-induced cardiac hypertrophy. eLife 2022, 11, e74519.
- Chen, Y.L.; Daneva, Z.; Kuppusamy, M.; Ottolini, M.; Baker, T.M.; Klimentova, E.; Shah, S.A.; Sokolowski, J.D.; Park, M.S.; Sonkusare, S.K. Novel Smooth Muscle Ca2+-Signaling Nanodomains in Blood Pressure Regulation. Circulation 2022, 146, 548–564.
- Li, S.S.; Gao, S.; Chen, Y.; Bao, H.; Li, Z.T.; Yao, Q.P.; Liu, J.T.; Wang, Y.; Qi, Y.X. Platelet-derived microvesicles induce calcium oscillations and promote VSMC migration via TRPV4. Theranostics 2021, 11, 2410–2423.
- Li, P.; Bian, X.; Liu, C.; Wang, S.; Guo, M.; Tao, Y.; Huo, B. STIM1 and TRPV4 regulate fluid flow-induced calcium oscillation at early and late stages of osteoclast differentiation. Cell Calcium 2018, 71, 45–52.
- Williams, K.M.; Leser, J.M.; Gould, N.R.; Joca, H.C.; Lyons, J.S.; Khairallah, R.J.; Ward, C.W.; Stains, J.P. TRPV4 calcium influx controls sclerostin protein loss independent of purinergic calcium oscillations. Bone 2020, 136, 115356.
- Suzuki, T.; Notomi, T.; Miyajima, D.; Mizoguchi, F.; Hayata, T.; Nakamoto, T.; Hanyu, R.; Kamolratanakul, P.; Mizuno, A.; Suzuki, M.; et al. Osteoblastic differentiation enhances expression of TRPV4 that is required for calcium oscillation induced by mechanical force. Bone 2013, 54, 172–178.
- Masuyama, R.; Vriens, J.; Voets, T.; Karashima, Y.; Owsianik, G.; Vennekens, R.; Lieben, L.; Torrekens, S.; Moermans, K.; Vanden Bosch, A.; et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008, 8, 257–265.
- Nam, M.H.; Park, H.J.; Seo, Y.K. Reduction of Osteoclastic Differentiation of Raw 264.7 Cells by EMF Exposure through TRPV4 and p-CREB Pathway. Int. J. Mol. Sci. 2023, 24, 3058.
- Lv, M.; Zhou, Y.; Chen, X.; Han, L.; Wang, L.; Lu, X.L. Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: Roles of calcium sources and cell membrane ion channels. J. Orthop. Res. 2018, 36, 730–738.
- Hurd, L.; Kirwin, S.M.; Boggs, M.; Mackenzie, W.G.; Bober, M.B.; Funanage, V.L.; Duncan, R.L. A mutation in TRPV4 results in altered chondrocyte calcium signaling in severe metatropic dysplasia. Am. J. Med. Genet. A 2015, 167, 2286–2293.
- Du, G.; Li, L.; Zhang, X.; Liu, J.; Hao, J.; Zhu, J.; Wu, H.; Chen, W.; Zhang, Q. Roles of TRPV4 and piezo channels in stretch-evoked Ca2+ response in chondrocytes. Exp. Biol. Med. 2020, 245, 180–189.
- Zhang, M.; Wu, X.; Du, G.; Chen, W.; Zhang, Q. Substrate stiffness-dependent regulatory volume decrease and calcium signaling in chondrocytes. Acta Biochim. Biophys. Sin. 2022, 54, 113–125.
- Kim, M.K.; Ramachandran, R.; Séguin, C.A. Spatiotemporal and functional characterisation of transient receptor potential vanilloid 4 (TRPV4) in the murine intervertebral disc. Eur. Cell Mater. 2021, 41, 194–203.
- Alessandri-Haber, N.; Dina, O.A.; Joseph, E.K.; Reichling, D.; Levine, J.D. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J. Neurosci. 2006, 26, 3864–3874.
- Kochukov, M.Y.; McNearney, T.A.; Yin, H.; Zhang, L.; Ma, F.; Ponomareva, L.; Abshire, S.; Westlund, K.N. Tumor necrosis factor-alpha (TNF-alpha) enhances functional thermal and chemical responses of TRP cation channels in human synoviocytes. Mol. Pain. 2009, 5, 49.
- O’Conor, C.J.; Ramalingam, S.; Zelenski, N.A.; Benefield, H.C.; Rigo, I.; Little, D.; Wu, C.L.; Chen, D.; Liedtke, W.; McNulty, A.L.; et al. Cartilage-Specific Knockout of the Mechanosensory Ion Channel TRPV4 Decreases Age-Related Osteoarthritis. Sci. Rep. 2016, 6, 29053.
- Hu, X.; Du, L.; Liu, S.; Lan, Z.; Zang, K.; Feng, J.; Zhao, Y.; Yang, X.; Xie, Z.; Wang, P.L.; et al. A TRPV4-dependent neuroimmune axis in the spinal cord promotes neuropathic pain. J. Clin. Investig. 2023, 133, e161507.
- Cui, Y.Y.; Li, M.Y.; Li, Y.T.; Ning, J.Y.; Gou, X.C.; Shi, J.; Li, Y.Q. Expression and functional characterization of transient receptor potential vanilloid 4 in the dorsal root ganglion and spinal cord of diabetic rats with mechanical allodynia. Brain Res. Bull. 2020, 162, 30–39.
- Jang, Y.; Jung, J.; Kim, H.; Oh, J.; Jeon, J.H.; Jung, S.; Kim, K.T.; Cho, H.; Yang, D.J.; Kim, S.M.; et al. Axonal neuropathy-associated TRPV4 regulates neurotrophic factor-derived axonal growth. J. Biol. Chem. 2012, 287, 6014–6024.
- Rodrigues, P.; Ruviaro, N.A.; Trevisan, G. TRPV4 Role in Neuropathic Pain Mechanisms in Rodents. Antioxidants 2022, 12, 24.
- Butenko, O.; Dzamba, D.; Benesova, J.; Honsa, P.; Benfenati, V.; Rusnakova, V.; Ferroni, S.; Anderova, M. The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS ONE 2012, 7, e39959.
- Jie, P.; Lu, Z.; Hong, Z.; Li, L.; Zhou, L.; Li, Y.; Zhou, R.; Zhou, Y.; Du, Y.; Chen, L.; et al. Activation of Transient Receptor Potential Vanilloid 4 is Involved in Neuronal Injury in Middle Cerebral Artery Occlusion in Mice. Mol. Neurobiol. 2016, 53, 8–17.
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trpv4-/- mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703.
- Eilert-Olsen, M.; Hjukse, J.B.; Thoren, A.E.; Tang, W.; Enger, R.; Jensen, V.; Pettersen, K.H.; Nagelhus, E.A. Astroglial endfeet exhibit distinct Ca2+ signals during hypoosmotic conditions. Glia 2019, 67, 2399–2409.
- Koide, M.; Wellman, G.C. Activation of TRPV4 channels does not mediate inversion of neurovascular coupling after SAH. Acta Neurochir. Suppl. 2015, 120, 111–116.
- Jung, C.; Fernández-Dueñas, V.; Plata, C.; Garcia-Elias, A.; Ciruela, F.; Fernández-Fernández, J.M.; Valverde, M.A. Functional coupling of GABA(A/B) receptors and the channel TRPV4 mediates rapid progesterone signaling in the oviduct. Sci. Signal 2018, 11, eaam6558.
- Jiang, P.; Li, S.S.; Xu, X.F.; Yang, C.; Cheng, C.; Wang, J.S.; Zhou, P.Z.; Liu, S.W. TRPV4 channel is involved in HSV-2 infection in human vaginal epithelial cells through triggering Ca2+ oscillation. Acta Pharmacol. Sin. 2023, 44, 811–821.
