Humic substances are naturally occurring materials composed of complex biogenic mixtures of substituted aromatic and aliphatic hydrocarbon core materials derived from the degradation and decomposition of dead plant and animal matter. They are ubiquitous in both terrestrial and aquatic systems constituting biotic pools and are characterized by unique properties; they are amphiphilic redox compounds with exceptional chelating features. Humic substances play a crucial role in both agriculture and the environment as carbon sequestrators, soil improvers, plant health promoters, as well as stabilizers of soil aggregates and regulators of organic/inorganic nutrients bioavailability.
- polymer characterization
- humic substances
- fulvic and humic acids
- textile dye
- dye adsorption
- molecular size
- chelation
- metal ion
1. Introduction
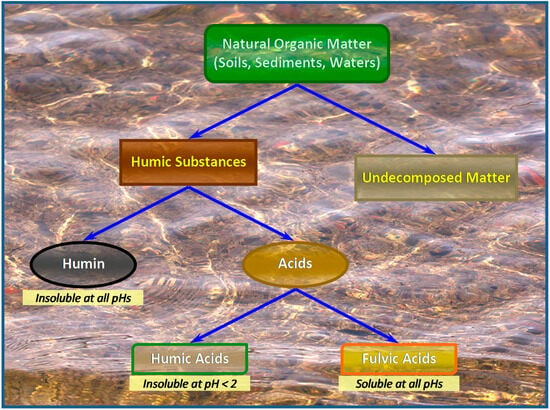
2. Structure and Characterization of Humic Substances
2.1. Macromolecular Nature and Chemical Retrosynthesis
2.2. Characterization Techniques, Supramolecules, and Molecular Weights
2.3. Structural and Compositional Architecture of Size Fractions
3. Interaction of Humic Substances with Soils and Pollutants
3.1. Soil Improvers, Chemical Regulators, and Chelating Agents
3.2. Complexation with Nutrient and Contaminant Metals
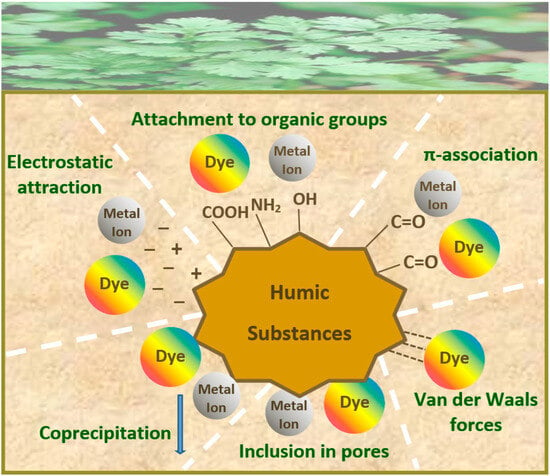
4. Dyestuff and Color Removal by Humic Substances
4.1. Dye Adsorption
4.2. Humic-Matrix Hybrid Materials in the Removal of Dyes
5. Conclusions
Humic substances are widely applied in agriculture as plant growth promoters exhibiting bactericidal and fungicidal properties—supporting both the sustainability of natural ecosystems and novel strategies for environmental protection—, in medicine, pharmaceutical, and cosmetic areas as solubilizing agents and to allow the transport of hydrophobic active compounds. Apart from that, the average properties of structurally heterogeneous HS, mostly their amphiphilic character and chelating functionality, have not yet been fully exploited and require further investigation. Future research may involve efforts to improve methods for the global standardization and chemical identification of HS. More importantly, much work should be undertaken to correlate the structural characteristics of HS molecules and fractions with the dye retention capacity; possible mechanisms for dye adsorption onto HS have not been thoroughly studied and must be confirmed on a case-by-case basis to establish specific tailored dye-pollution management systems. The diverse chemical and physical composition of HS is responsible for the facts that a single method cannot be utilized for the different HS–dye systems and that there is no universal mechanism that describes the manner in which HS–dye complexes are formed.
This entry is adapted from the peer-reviewed paper 10.3390/agronomy13122926
References
- Gaffney, J.S.; Marley, N.A.; Clark, S.B. Humic and fulvic acids and organic colloidal materials in the environment. In Humic and Fulvic Acids: Isolation, Structure, and Environmental Role; Gaffney, J.S., Marley, N.A., Clark, S.B., Eds.; American Chemical Society: Washington, DC, USA, 1996; p. 2.
- Rodríguez, F.J.; Schlenger, P.; García-Valverde, M. Monitoring changes in the structure and properties of humic substances following ozonation using UV–Vis, FTIR and 1H NMR techniques. Sci. Total Environ. 2016, 541, 623–637.
- Piccolo, A.; Nardi, S.; Concheri, G. Micelle-like conformation of humic substances as revealed by size exclusion chromatography. Chemosphere 1996, 33, 595–602.
- Piccolo, A.; Nardi, S.; Concheri, G. Macromolecular changes of humic substances induced by interaction with organic acids. Eur. J. Soil Sci. 1996, 47, 319–328.
- Barriquello, M.F.; Leite, F.L.; Deda, D.K.; Saab, S.D.; Consolin-Filho, N.; Piza, M.A.; Martin-Neto, L. Study of a model humic acid-type polymer by fluorescence spectroscopy and atomic force microscopy. Mater. Sci. Appl. 2012, 3, 478–484.
- Davies, G.; Ghabbour, E.A. (Eds.) Humic Substances: Structures, Properties and Uses; The Royal Society of Chemistry: London, UK, 1998; p. viii.
- Pokorná, L.; Gajdošová, D.; Mikeska, S.; Homoláč, P.; Havel, J. The stability of humic acids in alkaline media. In Humic Substances: Structures, Models and Functions; Ghabbour, E.A., Davies, G., Eds.; The Royal Society of Chemistry: London, UK, 2001; p. 133.
- Guetzloff, T.F.; Rice, J.A. Does humic acid form a micelle? Sci. Total Environ. 1994, 152, 31–35.
- Leboeuf, E.J.; Weber, W.J., Jr. Macromolecular characteristics of natural organic matter. 1. Insights from glass transition and enthalpic relaxation behavior. Environ. Sci. Technol. 2000, 34, 3623–3631.
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68.
- MacCarthy, P. The principles of humic substances: An introduction to the first principle. In Humic Substances: Structures, Models and Functions; Ghabbour, E.A., Davies, G., Eds.; The Royal Society of Chemistry: London, UK, 2001; p. 19.
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56.
- Ghabbour, E.A.; Davies, G. (Eds.) Humic Substances: Structures, Models and Functions; The Royal Society of Chemistry: London, UK, 2001; p. vii.
- Yang, F.; Antonietti, M. The sleeping giant: A polymer view on humic matter in synthesis and applications. Prog. Polym. Sci. 2020, 100, 101182.
- Mao, J.; Hu, W.; Schmidt-Rohr, K.; Davies, G.; Ghabbour, E.A.; Xing, B. Structure and elemental composition of humic acids: Comparison of solid-state 13C NMR calculations and chemical analyses. In Humic Substances: Structures, Properties and Uses; Davies, G., Ghabbour, E.A., Eds.; The Royal Society of Chemistry: London, UK, 1998; p. 79.
- Young, K.D.; Leboeuf, E.J. Glass transition behavior in a peat humic acid and an aquatic fulvic acid. Environ. Sci. Technol. 2000, 34, 4549–4553.
- Piccolo, A.; Conte, P.; Cozzolino, A. Chromatographic and spectrophotometric properties of dissolved humic substances compared with macromolecular polymers. Soil Sci. 2001, 166, 174–185.
- Cozzolino, A.; Piccolo, A. Polymerization of dissolved humic substances catalyzed by peroxidase. Effects of pH and humic composition. Org. Geochem. 2002, 33, 281–294.
- Durán, N.; Esposito, E. Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review. Appl. Catal. B 2000, 28, 83–99.
- Dec, J.; Bollag, J.-M. Phenoloxidase-mediated interactions of phenols and anilines with humic materials. J. Environ. Qual. 2000, 29, 665–676.
- Zou, J.; Huang, J.; Zhang, H.; Yue, D. Evolution of humic substances in polymerization of polyphenol and amino acid based on non-destructive characterization. Front. Environ. Sci. Eng. 2021, 15, 5.
- Diallo, M.S.; Faulon, J.-L.; Goddard, W.A.; Johnson, J.H., Jr. Binding of hydrophobic organic compounds to dissolved humic substances: A predictive approach based on computer assisted structure elucidation, atomistic simulations and Flory–Huggins solution theory. In Humic Substances: Structures, Models and Functions; Ghabbour, E.A., Davies, G., Eds.; The Royal Society of Chemistry: London, UK, 2001; p. 221.
- O’Loughlin, E.; Chin, Y.-P. Effect of detector wavelength on the determination of the molecular weight of humic substances by high-pressure size exclusion chromatography. Water Res. 2001, 35, 333–338.
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015.
- Schnitzer, M. Recent findings on the characterization of humic substances extracted from soils from widely differing climatic zones. In Soil Organic Matter Studies; IAEA: Vienna, Austria, 1977; Volume II, p. 117.
- Barth, H.G.; Boyes, B.E.; Jackson, C. Size exclusion chromatography. Anal. Chem. 1994, 66, 595R–620R.
- Chin, Y.-P.; Aiken, G.; O’Loughlin, E. Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ. Sci. Technol. 1994, 28, 1853–1858.
- Zhou, Q.; Cabaniss, S.E.; Maurice, P.A. Considerations in the use of high-pressure size exclusion chromatography (HPSEC) for determining molecular weights of aquatic humic substances. Water Res. 2000, 34, 3505–3514.
- Schäfer, A.I.; Mauch, R.; Waite, T.D.; Fane, A.G. Charge effects in the fractionation of natural organics using ultrafiltration. Environ. Sci. Technol. 2002, 36, 2572–2580.
- Chen, Y.; Senesi, N.; Schnitzer, M. Information provided on humic substances by E4/E6 ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358.
- Yan, S.; Zhang, N.; Li, J.; Wang, Y.; Liu, Y.; Cao, M.; Yan, Q. Characterization of humic acids from original coal and its oxidization production. Sci. Rep. 2021, 11, 15381.
- Rodríguez, F.J.; Núñez, L.A. Characterization of aquatic humic substances. Water Environ. J. 2011, 25, 163–170.
- Karpukhina, E.; Volkov, D.; Proskurnin, M. Quantification of lignosulfonates and humic components in mixtures by ATR FTIR spectroscopy. Agronomy 2023, 13, 1141.
- Liao, W.; Christman, R.F.; Johnson, J.D.; Millington, D.S.; Hass, J.R. Structural characterization of aquatic humic material. Environ. Sci. Technol. 1982, 16, 403–410.
- Enev, V.; Pospíšilová, L.; Klučáková, M.; Liptaj, T.; Doskočil, L. Spectral characterization of selected humic substances. Soil Water Res. 2014, 9, 9–17.
- Muscolo, A.; Sidari, M.; Cozzolino, V.; Nuzzo, A.; Nardi, S.; Piccolo, A. Molecular characteristics of humic substances from different origins and their effects on growth and metabolism of Pinus laricio callus. Chem. Biol. Technol. Agric. 2022, 9, 72.
- Polyakov, V.; Abakumov, E.V. Humic acids isolated from selected soils from the Russian Arctic and Antarctic: Characterization by two-dimensional 1H–13C HETCOR and 13C CP/Mas NMR spectroscopy. Geosciences 2020, 10, 15.
- Nebiosso, A.; Piccolo, A. Molecular rigidity and diffusivity of Al3+ and Ca2+ humates as revealed by NMR spectroscopy. Environ. Sci. Technol. 2009, 43, 2417–2424.
- Mao, J.-D.; Hu, W.-G.; Schmidt-Rohr, K.; Davies, G.; Ghabbour, E.A.; Xing, B. Quantitative characterization of humic substances by solid-state carbon-13 nuclear magnetic resonance. Soil Sci. Soc. Am. J. 2000, 64, 873–884.
- Rice, J.A.; MacCarthy, P. Statistical evaluation of the elemental composition of humic substances. Org. Geochem. 1991, 17, 635–648.
- da Silva, R.R.; Lucena, G.N.; Machado, Â.F.; de Freitas, G.A.; Matos, A.T.; Abrahão, W.A.P. Spectroscopic and elementary characterization of humic substances in organic substrates. Com. Sci. 2018, 9, 264–274.
- Shirshova, L.T.; Ghabbour, E.A.; Davies, G. Spectroscopic characterization of humic acid fractions isolated from soil using different extraction procedures. Geoderma 2006, 133, 204–216.
- Fukushima, M.; Tanaka, S.; Nakamura, H.; Ito, S. Acid–base characterization of molecular weight fractionated humic acid. Talanta 1996, 43, 383–390.
- Shin, H.-S.; Monsallier, J.M.; Choppin, G.R. Spectroscopic and chemical characterizations of molecular size fractionated humic acid. Talanta 1999, 50, 641–647.
- Christl, I.; Knicker, H.; Kögel-Knabner, I.; Kretzschmar, R. Chemical heterogeneity of humic substances: Characterization of size fractions obtained by hollow-fibre ultrafiltration. Eur. J. Soil Sci. 2000, 51, 617–625.
- Croué, J.-P. Isolation of humic and non-humic NOM fractions: Structural characterization. Environ. Monitor. Assess. 2004, 92, 193–207.
- Filella, M.; Buffle, J.; Parthasarathy, N. Humic and fulvic compounds. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Philadelphia, PA, USA, 2005; Volume 4, p. 288.
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974.
- Tiwari, J.; Ramanathan, A.; Bauddh, K.; Korstad, J. Humic substances: Structure, function and benefits for agroecosystems—A review. Pedosphere 2023, 33, 237–249.
- Hassett, D.J.; Bisesi, M.S.; Hartenstein, R. Bactericidal action of humic acids. Soil Biol. Biochem. 1987, 19, 111–113.
- Siddiqui, Y.; Meon, S.; Ismail, R.; Rahmani, M.; Ali, A. In vitro fungicidal activity of humic acid fraction from oil palm compost. Int. J. Agric. Biol. 2009, 11, 448–452.
- Prado, A.G.S.; Miranda, B.S.; Jacintho, G.V.M. Interaction of indigo carmine dye with silica modified with humic acids at solid/liquid interface. Surf. Sci. 2003, 542, 276–282.
- Hayes, M.H.B.; Malcolm, R.L. Considerations of compositions and of aspects of the structures of humic substances. In Humic Substances and Chemical Contaminants; Clapp, C.E., Ed.; SSSA: Madison, WI, USA, 2001; p. 3.
- Roulia, M. Humic substances: A novel eco-friendly fertilizer. Agronomy 2022, 12, 754.
- Alvarez-Puebla, R.A.; Goulet, P.J.G.; Garrido, J.J. Characterization of the porous structure of different humic fractions. Colloids Surf. A 2005, 256, 129–135.
- Burlakovs, J.; Kļaviņš, M.; Osinska, L.; Purmalis, O. The impact of humic substances as remediation agents to the speciation forms of metals in soil. APCBEE Procedia 2013, 5, 192–196.
- Zhong, X.; Yang, Y.; Liu, H.; Fang, X.; Zhang, Y.; Cui, Z.; Lv, J. New insights into the sustainable use of soluble straw humic substances for the remediation of multiple heavy metals in contaminated soil. Sci. Total Environ. 2023, 903, 166274.
- Zhou, S.; Chen, S.; Yuan, Y.; Lu, Q. Influence of humic acid complexation with metal ions on extracellular electron transfer activity. Sci. Rep. 2015, 5, 17067.
- Padhan, D.; Rout, P.P.; Kundu, R.; Adhikary, S.; Padhi, P.P. Bioremediation of heavy metals and other toxic substances by microorganisms. In Soil Bioremediation: An Approach towards Sustainable Technology; Parray, J.A., Abd Elkhalek Mahmoud, A.H., Sayyed, R., Eds.; Wiley: Hoboken, NJ, USA, 2021; p. 285.
- Perminova, I.V.; Hatfield, K. Remediation chemistry of humic substances: Theory and implications for technology. In Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice; Perminova, I.V., Hatfield, K., Hertkorn, N., Eds.; Springer: Dordrecht, The Netherlands, 2005; p. 3.
- Strawn, D.G.; Bohn, H.L.; O’Connor, G.A. Soil Chemistry, 5th ed.; Wiley: Hoboken, NJ, USA, 2020; p. 51.
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on aquatic flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802.
- Maheshwari, K.; Agrawal, M.; Gupta, A.B. Dye pollution in water and wastewater. In Novel Materials for Dye-Containing Wastewater Treatment; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2021; p. 1.
- Roulia, M.; Vassiliadis, A.A. Water purification by potassium humate–C.I. Basic Blue 3 adsorption-based interactions. Agronomy 2021, 11, 1625.
- Sheng, G.-P.; Zhang, M.-L.; Yu, H.-Q. Quantification of the interactions between a cationic dye and humic substances in aqueous solutions. J. Colloid Interface Sci. 2009, 331, 15–20.
- Sheng, G.-P.; Zhang, M.-L.; Yu, H.-Q. A rapid quantitative method for humic substances determination in natural waters. Anal. Chim. Acta 2007, 592, 162–167.
- Zanini, G.P.; Avena, M.J.; Fiol, S.; Arce, F. Effects of pH and electrolyte concentration on the binding between a humic acid and an oxazine dye. Chemosphere 2006, 63, 430–439.
- Hughes, D.J. Colorant for Foliage of Humic and/or Fulvic Acid, and Dye. U.S. Patent 7,431,743 B2, 7 October 2008.
- Hafuka, A.; Ding, Q.; Yamamura, H.; Yamada, K.; Satoh, H. Interactions of dissolved humic substances with oppositely charged fluorescent dyes for tracer techniques. Water Res. 2015, 85, 193–198.
- Basuki, R.; Rusdiarso, B.; Santosa, S.J.; Siswanta, D. The dependency of kinetic parameters as a function of initial solute concentration: New insight from adsorption of dye and heavy metals onto humic-like modified adsorbents. Bull. Chem. React. Eng. Catal. 2021, 16, 773–795.
- Gautam, R.K.; Tiwari, I. Humic acid functionalized magnetic nanomaterials for remediation of dye wastewater under ultrasonication: Application in real water samples, recycling and reuse of nanosorbents. Chemosphere 2020, 245, 125553.
- Sulistyaningsih, T.; Ariyani, S.; Astuti, W. Preparation of magnetite coated humic acid (Fe3O4–HA) as malachite green dye adsorbent. J. Phys. Conf. Ser. 2021, 1918, 032005.
- Abate, G.Y.; Alene, A.N.; Habte, A.T.; Addis, Y.A. Adsorptive removal of basic green dye from aqueous solution using humic acid modified magnetite nanoparticles: Kinetics, equilibrium and thermodynamic studies. J. Polym. Environ. 2021, 29, 967–984.
- Ahmad, N.; Arsyad, F.S.; Royani, I.; Lesbani, A. Selectivity of malachite green on cationic dye mixtures toward adsorption on magnetite humic acid. Environ. Nat. Resour. J. 2022, 20, 634–643.
- Luo, W.-J.; Gao, Q.; Wu, X.-L.; Zhou, C.-G. Removal of cationic dye (methylene blue) from aqueous solution by humic acid-modified expanded perlite: Experiment and theory. Sep. Sci. Technol. 2014, 49, 2400–2411.
- Volikov, A.B.; Ponomarenko, S.A.; Konstantinov, A.I.; Hatfield, K.; Perminova, I.V. Nature-like solution for removal of Direct Brown 1 azo dye from aqueous phase using humics-modified silica gel. Chemosphere 2016, 145, 83–88.
- Chassapis, K.; Roulia, M.; Vrettou, E.; Fili, D.; Zervaki, M. Biofunctional characteristics of lignite fly ash modified by humates: A new soil conditioner. Bioinorg. Chem. Appl. 2010, 2010, 457964.
