Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Hematology
Interleukins, a diverse family of cytokines produced by various cells, play crucial roles in immune responses, immunoregulation, and a wide range of physiological and pathological processes. In the context of megakaryopoiesis, thrombopoiesis, and platelet function, interleukins have emerged as key regulators, exerting significant influence on the development, maturation, and activity of megakaryocytes (MKs) and platelets.
- interleukins
- megakaryopoiesis
- thrombopoiesis
- bioengineering
1. Introduction
Megakaryocytes (MKs), mature hematopoietic cells derived from hematopoietic stem cells (HSCs), constitute a mere 0.05% of the total nucleated cells in bone marrow. Despite their scarcity, platelets play a pivotal role in a variety of physiological and pathological processes, including hemostasis, inflammation, and immunity [1]. The normal platelet counts in human blood range from 150 to 450 × 109/L. The human body releases approximately 100 billion platelets daily to maintain the standard quantity and functionality of platelets [2]. Any abnormality in this process of platelet homeostasis can severely impact the number or function of platelets, leading to platelet disorders [3]. Platelet disorders can be categorized into two main types based on their origin: acquired and inherited platelet disorders, which manifest as thrombocytosis, thrombocytopenia, and platelet function disorders. Acquired platelet disorders arise from external factors such as infections, medications, or autoimmune diseases, while inherited platelet disorders are caused by genetic mutations [4,5]. The severity of these disorders can vary dramatically in clinical settings. Even within the same type of disorder, the severity can range from negligible to life-threatening [6]. Some platelet disorders, due to their complexity and specificity, remain difficult to treat effectively as a universally effective cure has yet to be discovered [7]. The comprehensive study of the physiology of HSCs, their development into MKs, and subsequent platelet production and regulation is crucial for modern researchers to understand and investigate the corresponding pathological processes of platelet disorders. There are numerous fundamental studies in this area, and the traditional pathway of HSCs gradually developing into MKs in a homeostatic environment is largely understood. In recent years, the process of MK formation under dynamic and adaptive biological demands has been elucidated, revealing a non-classical pathway of MK development directly from HSCs [8]. These pathways are influenced and regulated by various cytokines, with interleukins playing a significant role.
2. The Role of Interleukins in Thrombopoiesis
The intricate process of platelet production, known as thrombopoiesis, is tightly regulated and involves the differentiation and maturation of MKs. It can be divided into three distinct stages. Initially, LT-HSCs are activated from a quiescent state to generate more active ST-HSCs; these then undergo a gradual loss of pluripotency and differentiate into MKPs via the stages of MPPs, CMPs, and MEPs. Subsequently, the progenitor cells proliferate and differentiate into immature MKs, which then undergo a series of maturation processes, such as an increase in DNA content, acquisition of surface proteins, and formation of the demarcation membrane system to generate mature MKs [41]. Finally, mature MKs either release platelets via a process called proplatelet formation or undergo rupture to release platelets directly into the bloodstream. Multiple cytokines, including thrombopoietin (TPO), play crucial roles in regulating thrombopoiesis at all stages, with TPO serving as the primary cytokine for regulating the process mentioned above [42]. Other cytokines, such as stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-SCF), stromal cell-derived factor 1 (SDF-1), and chemokine ligand 5 (CCL5), also contribute to the process of MK development and platelet production [43,44,45,46]. Interleukins, a family of signaling molecules, have emerged as critical regulators of thrombopoiesis. Interleukins such as IL-1, IL-3, and IL-6 promote the differentiation of HSCs into MKPs in the early stage of megakaryopoiesis, while IL-3, IL-6, and IL-11 facilitate the transition from immature to mature MKs [47,48]. Additionally, IL-1α can mediate MK rupture to accelerate platelet release in situations of a sudden platelet count decrease [49] (Figure 1). The intricacies of interleukin mechanisms in thrombopoiesis encompass interactions with other cytokines and signaling pathways. Specifically, interleukins IL-3, IL-6, and IL-11 require the presence of TPO and its receptor to effectively differentiate pro-megakaryocytes. These interleukins typically exhibit less efficacy individually compared to their synergistic performance with other cytokines, and their mechanisms of action are also associated with the three major pathways involved in TPO-mediated MK differentiation: JAK/STAT, PI3K/AKT, and MAPK/ERK [50,51,52].
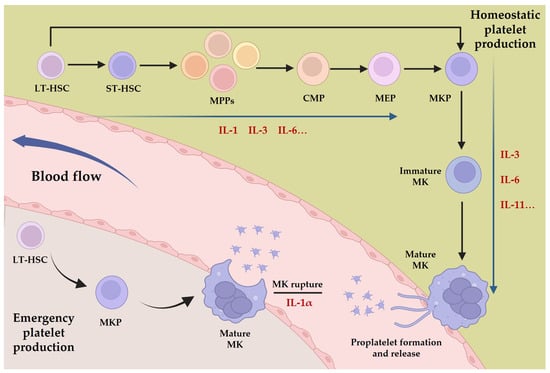
Figure 1. Platelet production and the interleukins involved in the process. Depending on the state of the body, platelets have two generative processes, homeostatic platelet production, and emergency platelet production, and interleukins are involved in different stages of these processes, such as IL-1, IL-3, IL-6, IL-11, etc.
While there is no evidence to suggest that interleukins are essential in homeostatic megakaryopoiesis, their significant regulatory role in megakaryopoiesis and the correlation between alterations in interleukin expression and changes in platelet count and function in non-homeostatic environments underscore their importance. In particular, IL-1α mediates the release of platelets via MK rupture during emergency platelet production, highlighting the unique influence of interleukins on the dynamics of megakaryopoiesis. Therefore, a thorough comprehension of the effects and mechanisms of interleukins on MK lineage cells is vital for comprehending cytokine regulation of thrombopoiesis in diverse organismal surroundings. This knowledge is expected to promote the progress of research and clinical application of cytokine therapies and biological agents.
3. Effects of Interleukins on Megakaryopoiesis and Platelet Function
In addition to the well-studied interleukins known to play a clear role in MK differentiation, several other interleukins have been identified as regulators of platelet production and function in vitro and in vivo. These interleukins have been classified within the interleukin family, and the sources of these regulatory interleukins in MKs, and their sources, along with their impact on platelet generation and function, are summarized in Table 1.
Table 1. Effects of interleukins on megakaryopoiesis and platelet function.
| Interleukins | Cell Sources | Effect on Megakaryopoiesis | Effect on Platelet Function | Refs. |
|---|---|---|---|---|
| IL-1 Family | ||||
| IL-1α | Macrophages Monocytes Microglia MKs, etc. |
Inducing MK rupture for rapid platelet production; inducing MK differentiation and maturation; inhibiting PPF formation. | / | [49] |
| IL-1β | Consistent with IL-1α | Promoting MK differentiation and maturation; inducing thrombopoiesis. | Enhancement of MK and platelet function. | [53,54] |
| IL-33 | Epithelial cells Necrotic cells Fibroblasts, etc. |
/ | Indirect promotion of platelet activation via 5-HT. | [55] |
| IL-37 | Monocytes Tonsil plasma cells Breast carcinoma cells, etc. |
/ | Inhibition of platelet activation and thrombosis in vivo. | [56] |
| IL-2 Family | ||||
| IL-2 | CD4+ and CD8+ T cells DCs NK and NKT cells, etc. |
Indirect increasing platelet counts in ITP patients; reducing platelet count in cancer patients. | Enhancement of platelet viability. | [57,58] |
| IL-4 | TH2 cells, Basophils, Eosinophils, Mast cells, etc. |
Inhibiting MK differentiation at all stages. | / | [59,60] |
| IL-9 | TH2, TH9, TH17 cells Treg cells Mast cells, etc. |
Promoting MK maturation and platelet production. | Promotion of platelet function and thrombosis | [61,62] |
| IL-21 | T cells NKT cells, etc. |
Promoting the proliferation of MKP and MK differentiation; increasing platelet clearance. | / | [63] |
| IL-3 Family | ||||
| IL-3 | T cells Macrophages NK cells Mast cells, etc. |
Promoting MKP proliferation and MK differentiation | / | [64] |
| IL-6 Family | ||||
| IL-6 | Monocytes/macrophages Endothelial cells Granulocytes Osteoblasts, etc. |
Promoting MK maturation and thrombopoiesis; enhancing the growth-promoting effect of TPO on MKs | Enhancement of canine platelet sensitivity to thrombin and platelet-activating factors | [65,66] |
| IL-11 | Bone marrow cells Epithelial cells Endothelial cells Osteoblasts, etc. |
Increasing bone marrow and splenic progenitor cell counts; promoting MK maturation to increase platelet counts | / | [67] |
| IL-17 Family | ||||
| IL-17A | TH17 cells CD8+ T cells NK cells, etc. |
Amplifying bone marrow-derived CFU-MK; regulating and synergizing the TPO/c-Mpl pathway for platelet function and factor release | / | [68,69,70] |
| Others | ||||
| IL-8 | Monocytes Macrophages Neutrophils Lymphocytes Endothelial cells, etc. |
Negative regulating MK lineage differentiation of HSPCs and proliferation of MKP | Negative regulation of platelet activation in patients with heparin-induced thrombocytopenia | [71,72] |
| IL-13 | NKT cells Mast cells Basophils Eosinophils, etc. |
Stimulating MK colony formation; promoting MK differentiation in HEL cells and Dami cells | / | [73] |
The impact of interleukins on the MK lineage cells can be broadly categorized into four aspects: their impact on MK differentiation, their role in platelet release, their effect on platelet function, and their involvement in platelet clearance (Figure 2). While most interleukins exert a promotive effect on MK differentiation and platelet function, a few have inhibitory effects. For instance, IL-1α enhances platelet release, and IL-4 inhibits MK differentiation. Notably, IL-2 has been observed to have variable effects on platelet counts under different pathological conditions. This suggests that interleukins can have diverse effects in different organismal environments. Therefore, comprehensively analyzing the underlying mechanisms of action is essential for understanding the pleiotropic effects of interleukins.
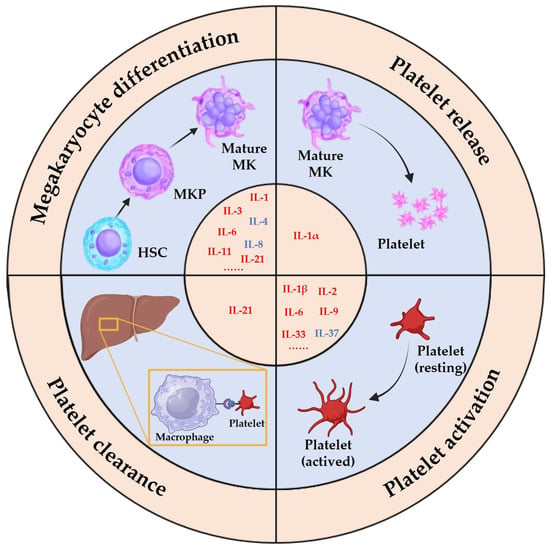
Figure 2. Effects of interleukins on megakaryocyte lineage cells. Interleukins have a variety of effects on megakaryocyte lineage cells in vivo and in vitro, which can be categorized into four main aspects: effects on megakaryocyte differentiation, platelet release, platelet clearance, and platelet function.
This entry is adapted from the peer-reviewed paper 10.3390/ph17010109
This entry is offline, you can click here to edit this entry!
