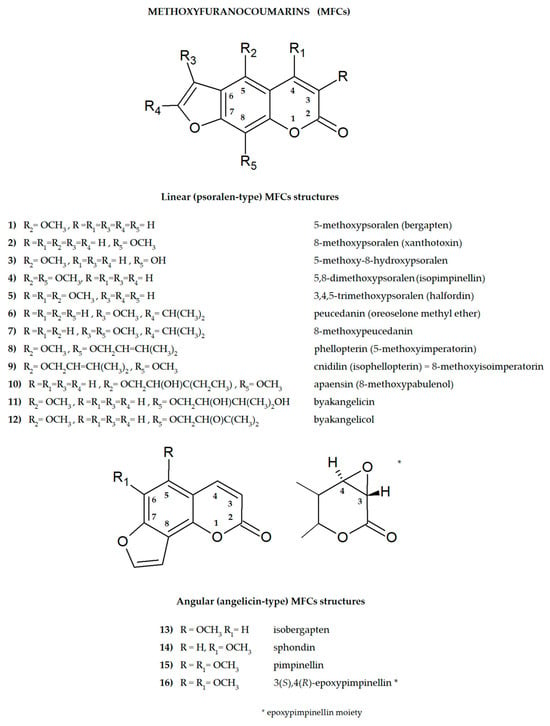
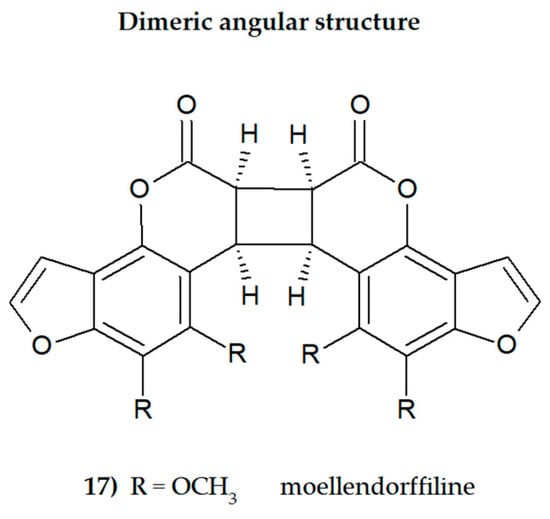
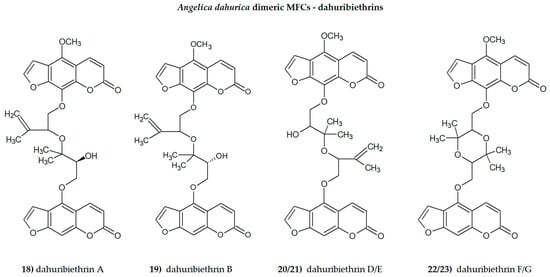
Plant secondary metabolites, including furanocoumarins, have attracted attention for decades as active molecules with therapeutic potential, especially those occurring in a limited number of species as evolutionarily specific and chemotaxonomically important. The most famous methoxyfuranocoumarins (MFCs), bergapten, xanthotoxin, isopimpinellin, phellopterin, byakangelicol, byakangelicin, isobergapten, pimpinellin, sphondin, as well as rare ones such as peucedanin and 8-methoxypeucedanin, apaensin, cnidilin, moellendorffiline and dahuribiethrins, have been investigated for their various biological activities.
- methoxyfuranocoumarins (MFCs)
- biological activity
- secondary metabolites
- plant drugs
1. Introduction
2. General Aspects of MFC Studies
2.1. Studies of the Active Substituents in the MFC Structure
2.2. Structure–Activity Relationships of the Studied MFCs—A Short Summary
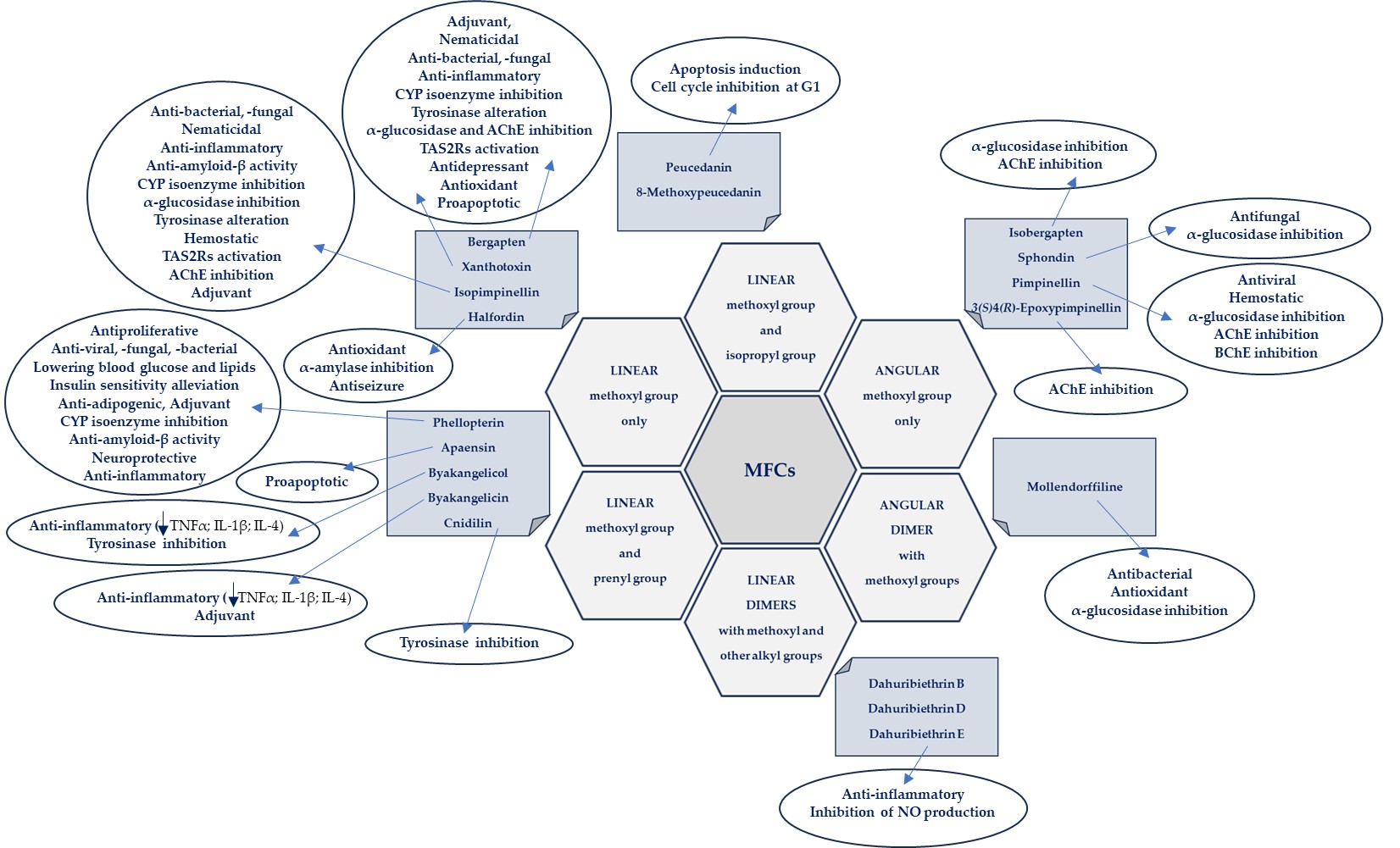
Some general observations regarding the structure–activity relationship of the analyzed MFCs could be concluded. The particular position of the methoxyl group and the count of these may influence the biological activity of linear MFCs. It was especially found when MFCs such as xanthotoxin, bergapten, and isopimpinellin as agonists of the bitter taste receptors were studied [89], where the specificity of the receptor target was also related to the position of the methoxy group in the furanocoumarin scaffold. The C-8 methoxy group was important for antiseizure activity of xanthotoxin, and bergapten with the C-5 methoxy group showed no protective properties [27]. In the case of α-glucosidase inhibitory activity [28] and antibacterial activity of MFCs [14], it was also important whether the molecule was in a linear or in an angular shape. Angular structures were more active (pimpinellin vs. isopimpinellin) and dimers were more active than monomers (moellendorffiline vs. pimpinellin).
The isopenthenyl group on the furanocoumarin skeleton increase their lipophilicity and the possibility of passage of the molecule through the bacterial membrane to its target site. Phellopterin possesses this group at the C-5 position. Double oxygenated substituents at C-5 and C-8 are necessary for antibacterial activity of linear furanocoumarins, such as in the case of isopimpinellin and phellopterin [29]. The latter one has a prenyl group, which increases lipophilicity, and therefore phellopterin was more active against bacterial strains than isopimpinellin [30]. Similar behavior was observed in the case of the adjuvant properties of byakangelicin, which increase the content of the co-ingested drugs in the brain by enhancing their penetration due to they own lipophilicity [31].
The presence of methoxy groups at C-8 and C-5 in the structure of furanocoumarin and the angular type of this structure are also beneficial for antifungal activity [32]. The analysis of the influence of methoxyl groups on the inhibition of AChE activity by angular MFCs indicated that pimpinellin (with two methoxy groups at C-5 and C-6 positions) exerted three-fold higher activity on AChE inhibition than isobergapten with one methoxy group at the C-5 position [33]. Pimpinellin was significantly (two-fold) more active compared to bergapten in the BChE inhibitory assay [34].
The furan ring fused at the C-6 and C-7 positions of the coumarin scaffold is necessary, and also the 5-methoxy group contributes greatly to reducing histamine release; however, the isopentane-derived groups in the molecule likely reduce histamine release as well [35]. Moellendorffiline (dimer of pimpinellin) exerts higher antioxidant activity compared to the monomeric compound, pimpinellin [28].
Inhibition of GABA-transaminase has been proposed as a possible mechanism for the antiepileptic effects of active MFCs. Analysis of the behavior of byakangelicin, byakangelicol and phellopterin (all these compounds have aliphatic chains at the C-8 position and are not active against GABA-transaminase) and in silico study showed that the three unblocked oxygen atoms in the MFC structure are necessary for the interaction of compounds with the enzyme pocket of GABA-transaminase. Therefore, if the compounds are to be active, the C-8 position cannot present an aliphatic chain [27].
All these observations are important, but many of them require additional, in-depth research, and this knowledge may be important when designing drugs based on MFC. It seems particularly interesting to examine the interaction of various substituents (methoxy, hydroxyl, isopropyl and prenyl groups) in the molecule, the position and number of which can modify activity. It should be taken into account that the impact of substituents may also depend on the selected research model and various factors, including physicochemical ones, and in the case of multi-component drugs, on the impact of co-existing molecules. All of this together makes this aspect even more difficult to explore, but it can also be an exciting challenge.
The biological activities of MFCs discussed in this text in relation to the structural type and substituents in the furanocoumarin molecule are briefly presented in Figure 4.
2.3. Bioavaiability Studies of MFCs
2.4. Distribution and Metabolism of MFCs
2.5. Gut Metabolism of MFCs by Human Microbiota
3. Toxicity and Safety Studies
As consumption of natural medicines continues to increase, attention should be paid to the potential risks associated with ingested bioactive compounds [92,93]. When developing new therapeutic strategies, including those related to natural medicines, safety of use (including toxicology) and assessment of side effects are an extremely important part of the related procedures. It has been demonstrated that toxicity induced by herbal medicines may result from the biotransformation of their components into electrophilic, reactive metabolites that can covalently bind to important macromolecules in the body. The complicated relationship between the active ingredients of natural drugs, their detoxification mechanisms and potential risks was highlighted in a recently published review article of Wen and Gorycki [47]. Furanocoumarins were also mentioned, metabolized to reactive toxic forms such as epoxides, cis-2-enedialdehyde, γ-ketoenal, and detoxified, the bioactivation of which may lead to the inactivation of drug metabolizing enzymes (including CYP isoenzymes); it clinically manifests itself through interactions and side effects when taking plant ingredients and synthetic drugs.
Bioactivation of the components of herbal extracts and the associated toxicity are largery governed by the complex composition of the said extracts [48], so understanding the complexity of natural medicines is of great importance [93]. As was underlined by Kharaman and co-investigators [48], the toxicity of a single compound can be reduced by interactions with accessory molecules present simultaneously in natural medicines. The different biological activities of these reactive metabolites can be viewed as a function of their reactivity, selectivity, concentration, and exposure time, as well as the pro-oxidant/antioxidant balance in cells [47].
A new approach to assessing the risk associated with the consumption of drugs and herbal preparations was proposed by Wang et al. [49] on the example of multi-component preparations of A. dahurica containing MFCs. It is worth undertaking and constantly developing this important aspect of the safety of natural medicines and functional foods [50][51].
4. New Approaches for Evaluation of MFC Activity
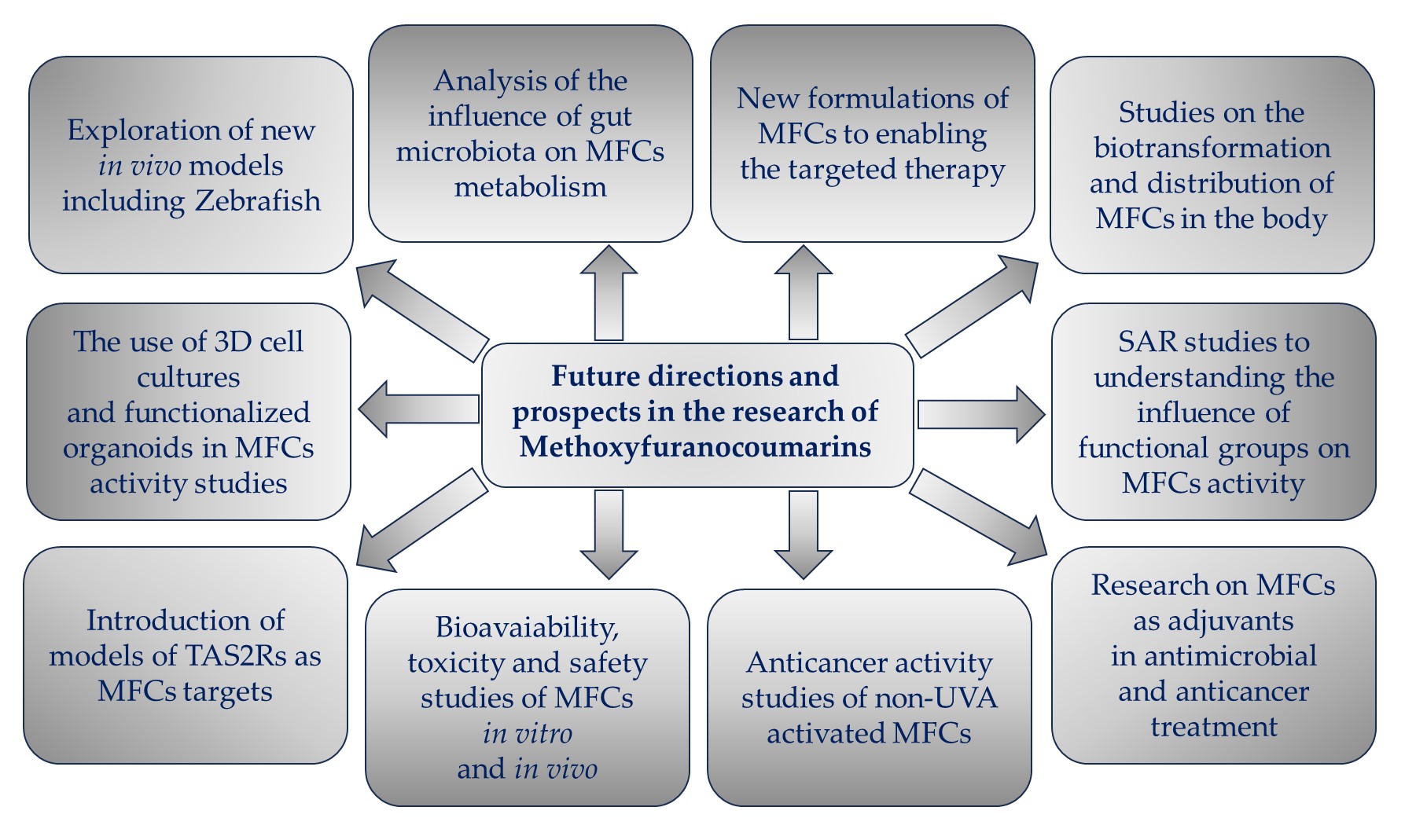
Figure 5. Future directions and prospects in the research of methoxyfuranocoumarins (MFCs).
This entry is adapted from the peer-reviewed paper 10.3390/cimb46010055
References
- Limones-Mendez, M.; Dugrand-Judek, A.; Villard, C.; Coqueret, V.; Froelicher, Y.; Bourgaud, F.; Olry, A.; Hehn, A. Convergent evolution leading to the appearance of furanocoumarins in citrus plants. Plant Sci. 2020, 292, 110392.
- Fernandes, H.P.; Salome-Abarca, L.F.; Goncalves Pereira, R.; Brandao Seibert, J.; Jose-Silva Junior, G.; Das Gracas Fernandes da Silva, M.F.; Choi, J.H. Metabolomic investigation of Citrus latifolia and the putative role of coumarins in resistance to black spot disease. Front. Mol. Biosci. 2022, 9, 934401.
- Zhu, J.J.; Jiang, J.G. Pharmacological and Nutritional Effects of Natural Coumarins and Their Structure-Activity Relationships. Mol. Nutr. Food Res. 2018, 62, e1701073.
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622.
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Pons-Fuster Lopez, E.; Nidaa Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346.
- Liang, Y.; Xie, L.; Liu, K.; Cao, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. Bergapten: A review of its pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2021, 35, 6131–6147.
- Wu, A.; Lu, J.; Zhong, G.; Lu, L.; Qu, Y.; Zhang, C. Xanthotoxin (8-methoxypsoralen): A review of its chemistry, pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2022, 36, 3805–3832.
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413.
- Murray, R.D.H.; Mendez, J.; Brown, S.A. The Natural Coumarins: Occurrence, Chemistry and Biochemistry; Willey: Chichester, UK, 1982; p. 702.
- Brown, S.A. Biosynthesis of furanocoumarins in parsnips. Phytochemistry 1970, 9, 2471–2475.
- Stanjek, V.; Piel, J.; Boland, W. Biosynthesis of furanocoumarins: Mevalonate-independent prenylation of umbelliferone in Apium graveolens (Apiaceae). Phytochemistry 1999, 50, 1141–1146.
- Villard, C.; Munakata, R.; Kitajima, S.; Velzen, R.; Schranz, E.M.; Larbat, R.; Hehn, A. A new P450 involved in the furanocoumarin pathway underlies a recent case of convergent evolution. New Phytol. 2021, 231, 1923–1939.
- Yang, W.-Q.; Song, Y.-L.; Zhu, Z.-X.; Su, C.; Zhang, X.; Wang, J.; Shi, S.-P.; Tu, P.-F. Anti-inflammatory dimeric furanocoumarins from the roots of Angelica dahurica. Fitoterapia 2015, 105, 187–193.
- Dehghan, H.; Rezaee, P.; Aliahmadi, A. Bioassay screening of 12 Iranian plants and detection of antibacterial compounds from Heracleum persicum using a TLC bioautography method. J. Liquid Chrom. Rel. Technol. 2020, 43, 381–387.
- Lin, S.R.; Chang, C.H.; Tsai, M.J.; Cheng, H.; Chen, J.C.; Leong, M.K.; Weng, C.F. The perceptions of natural compounds against dipeptidyl peptidase 4 in diabetes: From in silico to in vivo. Ther. Adv. Chronic Dis. 2019, 10, 2040622319875305.
- Bartnik, M.; Sławińska-Brych, A.; Żurek, A.; Kandefer-Szerszeń, M.; Zdzisińska, B. 8-methoxypsoralen reduces AKT phosphorylation, induces intrinsic and extrinsic apoptotic pathways, and suppresses cell growth of SK-N-AS neuroblastoma and SW620 metastatic colon cancer cells. J. Ethnopharmacol. 2017, 207, 19–29.
- Bartnik, M.; Sławińska-Brych, A.; Mizerska-Kowalska, M.; Zdzisińska, B. Evaluation of the Biological Effect of Non-UV-Activated Bergapten on Selected Human Tumor Cells and the Insight into the Molecular Mechanism of Its Action. Int. J. Mol. Sci. 2023, 24, 15555.
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423.
- Madeiro, S.A.L.; Borges, N.H.P.B.; Souto, A.L.; de Figueiredo, P.T.R.; Siqueira-Junior, J.P.; Tavares, J.F. Modulation of the antibiotic activity against multidrug resistant strains of coumarins isolated from Rutaceae species. Microb. Pathog. 2017, 104, 151–154.
- Skalicka-Woźniak, K.; Orhan, I.E.; Cordell, G.A.; Nabavi, S.M.; Budzyńska, B. Implication of Coumarins towards Central Nervous System Disorders. Pharmacol. Res. 2016, 103, 188–203.
- Skiba, A.; Kozioł, E.; Luca, S.V.; Budzyńska, B.; Podlasz, P.; Van Der Ent, W.; Shojaeinia, E.; Esguerra, C.V.; Nour, M.; Marcourt, L.; et al. Evaluation of the Antiseizure Activity of Endemic Plant Halfordia kendack Guillaumin and Its Main Constituent, Halfordin, on a Zebrafish Pentylenetetrazole (PTZ)-Induced Seizure Model. Int. J. Mol. Sci. 2023, 24, 2598.
- John, D.R.; Marjorie, C.C. Basic Principles of Organic Chemistry, 2nd ed.; W. A. Benjamin, Inc.: Menlo Park, CA, USA, 1977; ISBN 0-8053-8329-8. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Basic_Principles_of_Organic_Chemistry_(Roberts_and_Caserio)/26%3A_More_on_Aromatic_Compounds/26.06%3A_Correlations_of_Structure_with_Reactivity_of_Aromatic_Compounds (accessed on 24 November 2023).
- Türker, L. Monomethoxyisomers of psoralen—DFT treatment. Earthline J. Chem. Sci. 2022, 8, 175–192.
- Lake, B.G. Coumarin metabolism, toxicity, and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453.
- Born, S.; Rodriquez, P.; Eddy, C.; Lehman-McKeeman, L. Synthesis and reactivity of coumarin 3,4-epoxide. Drug Metab. Dispos. 1997, 25, 1318–1323.
- Karamat, F.; Olry, A.; Munakata, R.; Koeduka, T.; Sugiyama, A.; Paris, C.; Hehn, A.; Bourgaud, F.; Yazaki, K. A coumarin-specific prenyltransferase catalyzes the crucial biosynthetic reaction for furanocoumarin formation in parsley. Plant J. 2014, 77, 627–638.
- Kozioł, E.; Jóźwiak, K.; Budzyńska, B.; de Witte, P.A.M.; Copmans, D.; Skalicka-Woźniak, K. Comparative Antiseizure Analysis of Diverse Natural Coumarin Derivatives in Zebrafish. Int. J. Mol. Sci. 2021, 22, 11420.
- Dehghan, H.; Sarrafi, Y.; Salehi, P.; Ebrahimi, S.N. α-Glucosidase inhibitory and antioxidant activity of furanocoumarins from Heracleum persicum. Med. Chem. Res. 2017, 26, 849–855.
- Walasek, M.; Grzegorczyk, A.; Malm, A.; Skalicka-Woźniak, K. Bioactivity-guided isolation of antimicrobial coumarins from Heracleum mantegazzianum Sommier & Levier (Apiaceae) fruits by high-performance counter-current chromatography. Food Chem. 2015, 186, 133–138.
- Zuo, G.-Y.; Wang, C.-J.; Han, J.; Li, Y.-Q.; Wang, G.-C. Synergism of coumarins from Chinese drug Zanthoxylum nitidum with antibacterial agents against methicillin-resistent Staphylococcus aureus (MRSA). Phytomedicine 2016, 23, 1814–1820.
- Kang, Y.Y.; Song, J.; Kim, J.Y.; Jung, H.; Yeo, W.-S.; Lim, Y.; Mok, H. Byakangelicin as a modulator for improved distribution and bioactivity of natural compounds and synthetic drugs in the brain. Phytomed 2019, 62, 152963.
- He, Y.-H.; Shang, X.-F.; Li, H.-X.; Li, A.-P.; Tang, C.; Zhang, B.-Q.; Zhang, Z.-J.; Wang, R.; Ma, Y.; Du, S.-S.; et al. Antifungal activity and action mechanism study of coumarins from Cnidium monnieri fruit and structurally related compounds. Chem. Biodiv. 2021, 18, e2100633.
- Lee, S.J.; Depoortere, I.; Hatt, H. Therapeutic potential of ectopic olfactory and taste receptors. Nat. Rev. Drug Discov. 2019, 18, 116–138.
- Karakaya, S.; Koca, M.; Yılmaz, S.V.; Yıldırım, K.; Pınar, N.M.; Demirci, B.; Brestic, M.; Sytar, O. Molecular Docking Studies of Coumarins Isolated from Extracts and Essential Oils of Zosima absinthifolia Link as Potential Inhibitors for Alzheimer’s Disease. Molecules 2019, 24, 722.
- Li, D.; Wu, L. Coumarins from the roots of Angelica dahurica cause anti-allergic inflammation. Exper. Ther. Med. 2017, 14, 874–880.
- Kulikov, O.A.; Ageev, V.P.; Brodovskaya, E.P.; Shlyapkina, V.I.S.; Petrov, P.S.; Zharkov, M.N.; Yakobson, D.E.; Maev, I.V.; Sukhorukov, G.B.; Pyataev, A.N. Evaluation of photocytotoxicity liposomal form of furanocoumarins Sosnowsky’s hogweed. Chem.-Biol. Interact. 2022, 357, 109880.
- Petit, C.; Bujard, A.; Skalicka-Woźniak, K.; Cretton, S.; Houriet, J.; Christen, P.; Carrupt, P.-A.; Wolfender, J.-L. Prediction of the passive intestinal absorption of medicinal plant extract constituents with the parallel artificial membrane permeability assay (PAMPA). Planta Med. 2016, 82, 424–431.
- Li, H.; Zeng, H.; He, D.; Wang, M.; Liu, L.; Liang, W.; Shu, Y.; Zhao, S.; Sun, G.; Lv, C.; et al. A new approach to examining the extracton process of Zhishi and Zhiqiao considering the synergistic effect of complex mixtures by PAMPA. J. Chrom. B 2018, 1099, 10–17.
- Zhao, A.-H.; Zhang, Y.-B.; Yang, X.-W. Simultaneous determination, and pharmacokinetics of sixteen Angelicae dahurica coumarins in vivo by LC–ESI-MS/MS following oral delivery in rats. Phytomedicine 2016, 23, 1029–1036.
- Liao, M.; Song, G.; Cheng, X.; Diao, X.; Sun, Y.; Zhang, L. Simultaneous determination of six coumarins in rat plasma and metabolites identification of bergapten in vitro and in vivo. J. Agric. Food Chem. 2018, 66, 4602–4613.
- Zhang, Y.B.; Deng, G.G.; Wang, T.X.; Liu, L.; Yang, X.W. Tissue distribution study of Angelica dahurica cv. Yubaizhi in rat by ultra-performance liquid chromatography with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 174, 43–49.
- Qiu, J.; Zhu, M.; Wang, Y.; Chen, B.; Bai, R.; Chen, F.; Li, Y.; Zhou, Y.; Zhang, L. Pharmacokinetic and excretion study of eight active constituents in rat by LC-MS/MS after oral administration of the Toddalia asiatica extract. Anal. Biochem. 2022, 640, 114407.
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e0033820.
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001.
- Susanto Tan, S.R.; Eser, B.E.; Han, J. Gut metabolism of furanocoumarins: Proposed function of Co O-methyltransferase. ACS Omega 2020, 5, 30696–30703.
- Kim, M.; Kim, N.; Han, J. Metabolism od Kaempferia parviflora polymethoxyflavones by human intestinal bacterium, Blautia sp. MRG-PMF1. J. Agric. Food. Chem. 2014, 62, 12377–12383.
- Wen, B.; Gorycki, P. Bioactivation of herbal constituents: Mechanisms and toxicological relevance. Drug Metab. Rev. 2019, 51, 453–497.
- Kahraman, C.; Ceren Arituluk, Z.; Irem Tatli Cankaya, I. The Clinical Importance of Herb-Drug Interactions and Toxicological Risks of Plants and Herbal Products. In Medical Toxicology; IntechOpen: London, UK, 2021.
- Wang, Z.; Zan, K.; Hu, X.-W.; Kang, S.; Li, H.-L.; Zuo, T.-T.; Jin, H.-Y.; Ma, S.-C. The Simultaneous Determination of Nine Furocoumarins in Angelica dahurica Using UPLC Combined with the QAMS Approach and Novel Health Risk Assessment Based on the Toxic Equivalency Factor. Separations 2023, 10, 508.
- Yordi, E.G.; Matos, M.J.; Martinez, A.P.; Tornes, A.C.; Santana, L.; Molina, E.; Uriarte, E. In silico genotoxicity of coumarins: Application of the Phenol-Explorer food database to functional food science. Food Funct. 2017, 8, 2958–2966.
- Zhang, Y.; Li, Z.; Wei, J.; Kong, L.; Song, M.; Zhang, Y.; Xiao, X.; Cao, H.; Jin, Y. Network pharmacology and molecular docking reveal the mechanism of Angelica dahurica against Osteosarcoma. Medicine 2022, 101, e31055.
- Yerer, M.B.; Dayan, S.; Han, M.I.; Sharma, A.; Tuli, H.S.; Sak, K. Nanoformulations of coumarins and the hybrid molecules of coumarins with potential anticancer effects. Anticancer Agents Med. Chem. 2020, 20, 1797–1816.
- Wu, J.Y.; Li, Y.J.; Liu, T.T.; Ou, G.; Hu, X.B.; Tang, T.T.; Wang, J.M.; Liu, X.Y.; Xiang, D.X. Microemulsions vs chitosan derivative-coated microemulsions for dermal delivery of 8-methoxypsoralen. Int. J. Nanomed. 2019, 14, 2327–2340.
- Del Dosso, A.; Urenda, J.; Nguyen, T.; Quadrato, G. Upgrading the Physiological Relevance of Human Brain Organoids. Neuron 2020, 107, 1014–1028.
- D’Antoni, C.; Mautone, L.; Sanchini, C.; Tondo, L.; Grassmann, G.; Cidonio, G.; Bezzi, P.; Cordella, F.; Di Angelantonio, S. Unlocking Neural Function with 3D In Vitro Models: A Technical Review of Self-Assembled, Guided, and Bioprinted Brain Organoids and Their Applications in the Study of Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2023, 24, 10762.
- Steimberg, N.; Chiono, V.; Era, P.D.; Di Angelantonio, S. iPS, Organoids and 3D Models as Advanced Tools for In Vitro Toxicology. ALTEX Altern. Anim. Exp. 2020, 37, 136–140.
- Ki, S.Y.; Jeong, Y.T. Taste Receptors beyond Taste Buds. Int. J. Mol. Sci. 2022, 23, 9677.
- Qin, C.; Yuan, Q.; Han, H.; Chen, C.; Wu, J.; Wei, X.; Liu, M.; Zhang, H.; Ping, J.; Xu, L.; et al. Biomimetic integrated gustatory and olfactory sensing array based on HL-1 cardiomyocyte facilitating drug screening for tachycardia treatment. Biosens. Bioelectr. 2023, 223, 115034.
