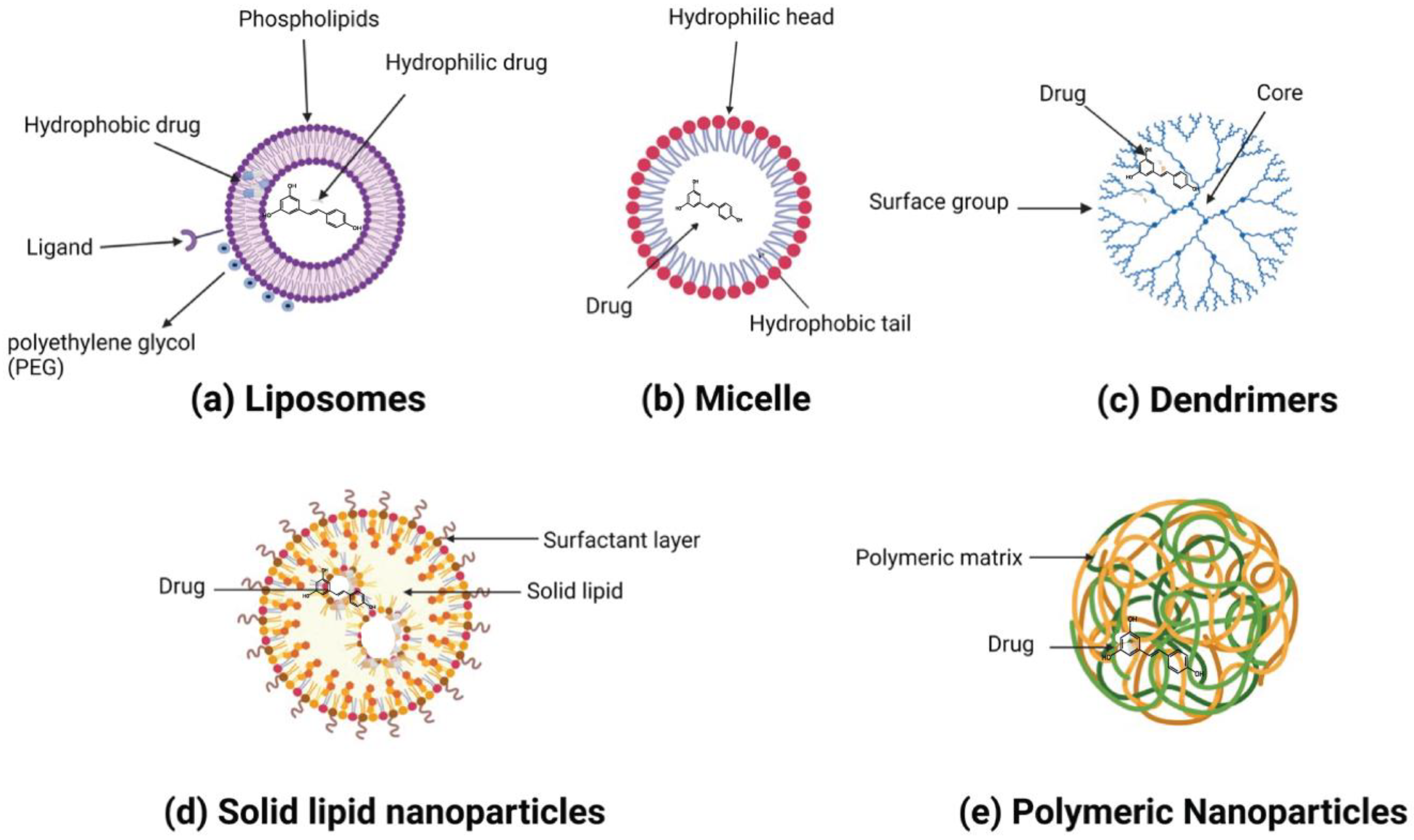
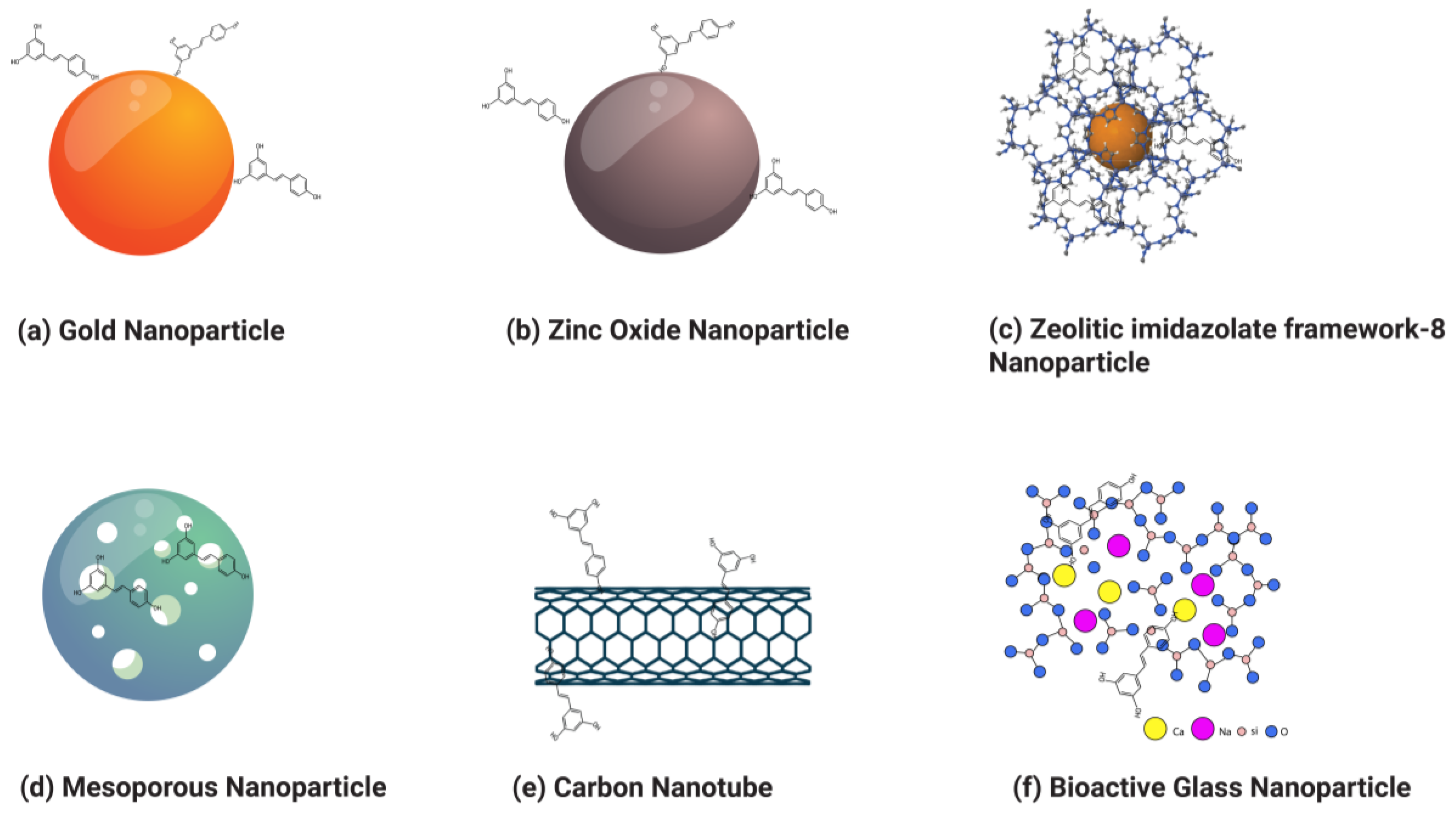
Resveratrol is a polyphenolic compound that has gained considerable attention in the past decade due to its multifaceted therapeutic potential, including anti-inflammatory and anticancer properties. However, its anticancer efficacy is impeded by low water solubility, dose-limiting toxicity, low bioavailability, and rapid hepatic metabolism. To overcome these hurdles, various nanoparticles such as organic and inorganic nanoparticles, liposomes, polymeric nanoparticles, dendrimers, solid lipid nanoparticles, gold nanoparticles, zinc oxide nanoparticles, zeolitic imidazolate frameworks, carbon nanotubes, bioactive glass nanoparticles, and mesoporous nanoparticles were employed to deliver resveratrol, enhancing its water solubility, bioavailability, and efficacy against various types of cancer.
- anticancer properties
- bioavailability
- nanoparticles
- polyphenolic compound
- resveratrol encapsulation
1. Introduction
2. Resveratrol
2.1. The Structure and Physical Properties of Resveratrol
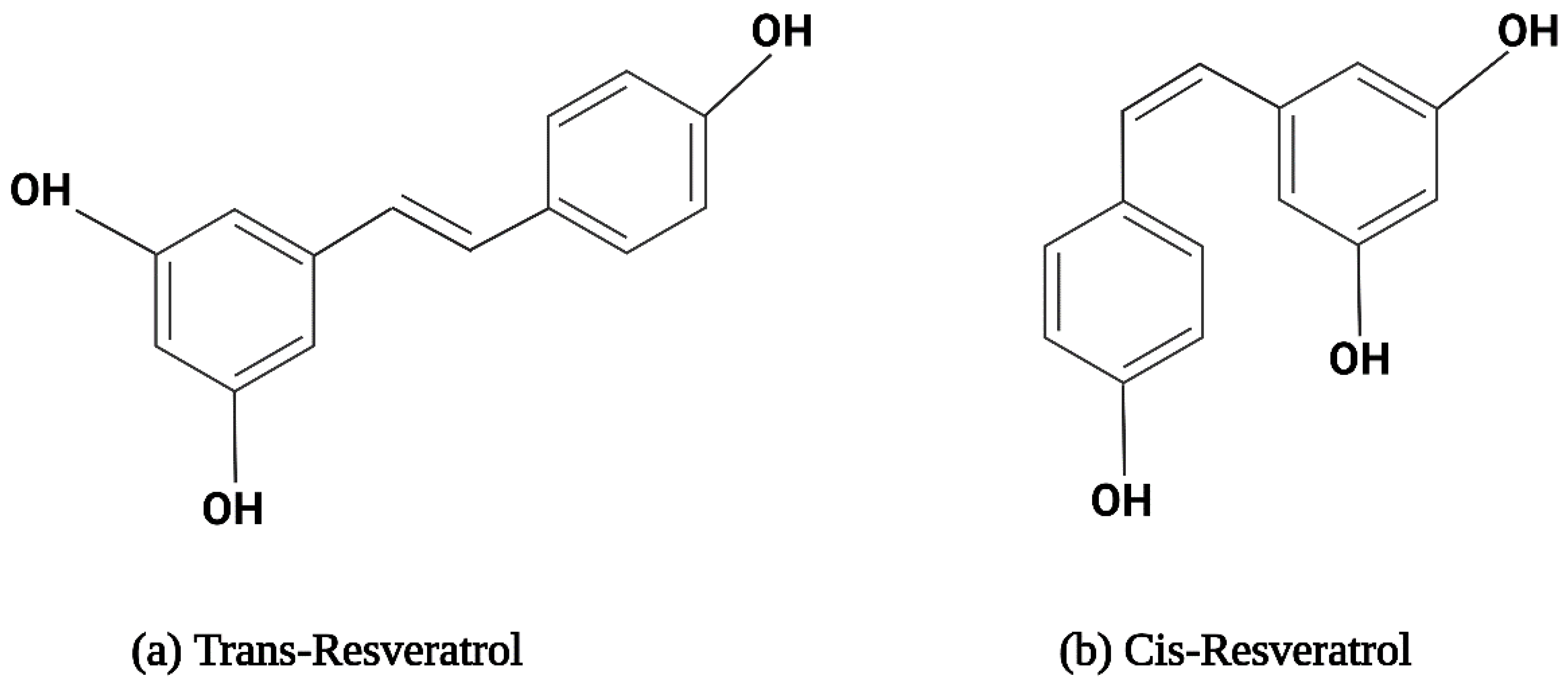
2.2. Metabolism of Resveratrol
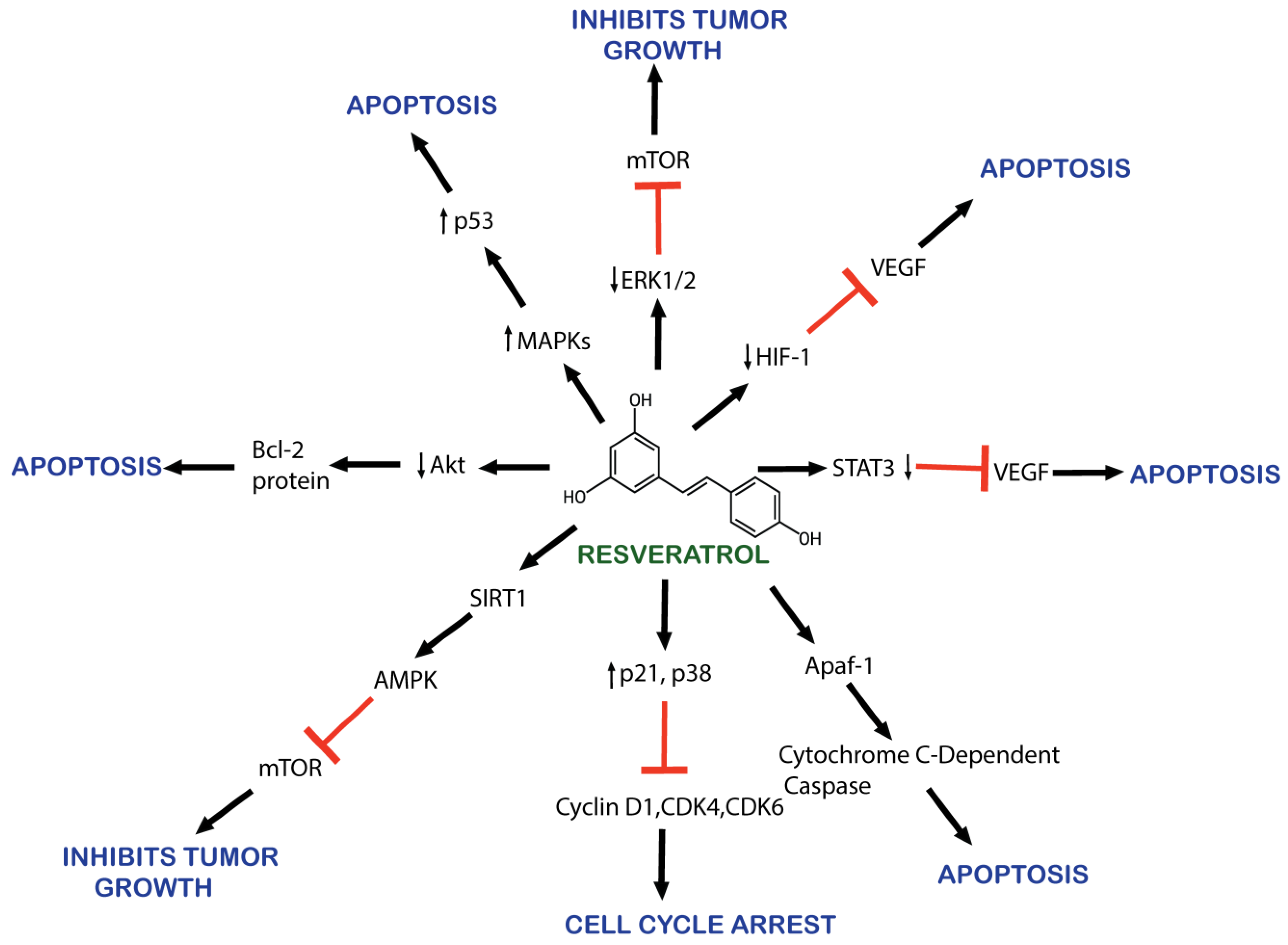
2.3. Mechanism of Action of Resveratrol against Cancer
3. Application of Nanoparticles to Improve the Therapeutic Potential of Resveratrol for Cancer
This entry is adapted from the peer-reviewed paper 10.3390/ph17010126
References
- Sleeman, K.E.; de Brito, M.; Etkind, S.; Nkhoma, K.; Guo, P.; Higginson, I.J.; Gomes, B.; Harding, R. The Escalating Global Burden of Serious Health-Related Suffering: Projections to 2060 by World Regions, Age Groups, and Health Conditions. Lancet Glob. Health 2019, 7, e883–e892.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories from 2020 to 2050. JAMA Oncol. 2023, 9, 465.
- Mathan, S.V.; Rajput, M.; Singh, R.P. CHAPTER 14—Chemotherapy and Radiation Therapy for Cancer. In Understanding Cancer; Jain, B., Pandey, S., Eds.; Academic Press: New York, NY, USA, 2022; pp. 217–236. ISBN 978-0-323-99883-3.
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199.
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7.
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer Treatment and Survivorship Statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289.
- Nagori, K.; Nakhate, K.T.; Yadav, K.; Ajazuddin; Pradhan, M. Unlocking the Therapeutic Potential of Medicinal Plants for Alzheimer’s Disease: Preclinical to Clinical Trial Insights. Future Pharmacol. 2023, 3, 877–907.
- Singh Purewal, S.; Punia Bangar, S.; Kaur, P. (Eds.) Recent Advances in Citrus Fruits; Springer International Publishing: Cham, Switzerland, 2023; ISBN 978-3-031-37533-0.
- El-Saadony, M.T.; Zabermawi, N.M.; Zabermawi, N.M.; Burollus, M.A.; Shafi, M.E.; Alagawany, M.; Yehia, N.; Askar, A.M.; Alsafy, S.A.; Noreldin, A.E. Nutritional Aspects and Health Benefits of Bioactive Plant Compounds against Infectious Diseases: A Review. Food Rev. Int. 2023, 39, 2138–2160.
- Adetuyi, B.O.; Odelade, K.A.; Odine, G.O.; Adetuyi, O.A.; Omowumi, S.O.; Ogunlana, O.O.; Egbuna, C. Neurorestorative Potential of Medicinal Plants and Their Phytochemicals. In Phytochemical Drug Discovery for Central Nervous System Disorders; Wiley: Hoboken, NJ, USA, 2023; pp. 291–310. ISBN 978-1-119-79412-7.
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324.
- Gorain, B.; Karmakar, V.; Sarkar, B.; Dwivedi, M.; Leong, J.T.L.; Toh, J.H.; Seah, E.; Ling, K.Y.; Chen, K.Y.; Choudhury, H.; et al. Biomacromolecule-Based Nanocarrier Strategies to Deliver Plant-Derived Bioactive Components for Cancer Treatment: A Recent Review. Int. J. Biol. Macromol. 2023, 253, 126623.
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077.
- Islam, M.R.; Jony, M.H.; Thufa, G.K.; Akash, S.; Dhar, P.S.; Rahman, M.M.; Afroz, T.; Ahmed, M.; Hemeg, H.A.; Rauf, A.; et al. A Clinical Study and Future Prospects for Bioactive Compounds and Semi-Synthetic Molecules in the Therapies for Huntington’s Disease. Mol. Neurobiol. 2023.
- Gosline, G.; Bidault, E.; van der Burgt, X.; Cahen, D.; Challen, G.; Condé, N.; Couch, C.; Couvreur, T.L.; Dagallier, L.-P.M.; Darbyshire, I. A Taxonomically-Verified and Vouchered Checklist of the Vascular Plants of the Republic of Guinea. Sci. Data 2023, 10, 327.
- Schultz, F.; Garbe, L. How to Approach a Study in Ethnopharmacology? Providing an Example of the Different Research Stages for Newcomers to the Field Today. Pharmacol. Res. Perspect. 2023, 11, e01109.
- Cadoná, F.C.; Dantas, R.F.; de Mello, G.H.; Silva, F.P., Jr. Natural Products Targeting into Cancer Hallmarks: An Update on Caffeine, Theobromine, and (+)-Catechin. Crit. Rev. Food Sci. Nutr. 2022, 62, 7222–7241.
- Priya, S.; Satheeshkumar, P. Natural Products from Plants: Recent Developments in Phytochemicals, Phytopharmaceuticals, and Plant-Based Neutraceuticals as Anticancer Agents. Funct. Preserv. Prop. Phytochem. 2020, 145–163.
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803.
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896.
- Bernitsa, S.; Dayan, R.; Stephanou, A.; Tzvetanova, I.D.; Patrikios, I.S. Natural Biomolecules and Derivatives as Anticancer Immunomodulatory Agents. Front. Immunol. 2023, 13, 1070367.
- Jang, J.-H.; Lee, T.-J. Mechanisms of Phytochemicals in Anti-Inflammatory and Anti-Cancer. Int. J. Mol. Sci. 2023, 24, 7863.
- Majrashi, T.A.; Alshehri, S.A.; Alsayari, A.; Muhsinah, A.B.; Alrouji, M.; Alshahrani, A.M.; Shamsi, A.; Atiya, A. Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients 2023, 15, 1704.
- Sohel, M.; Aktar, S.; Biswas, P.; Amin, M.A.; Hossain, M.A.; Ahmed, N.; Mim, M.I.H.; Islam, F.; Mamun, A.A. Exploring the Anti-cancer Potential of Dietary Phytochemicals for the Patients with Breast Cancer: A Comprehensive Review. Cancer Med. 2023, 12, 14556–14583.
- Dogra, A.; Kumar, J. Biosynthesis of Anticancer Phytochemical Compounds and Their Chemistry. Front. Pharmacol. 2023, 14, 1136779.
- Gahtori, R.; Tripathi, A.H.; Kumari, A.; Negi, N.; Paliwal, A.; Tripathi, P.; Joshi, P.; Rai, R.C.; Upadhyay, S.K. Anticancer Plant-Derivatives: Deciphering Their Oncopreventive and Therapeutic Potential in Molecular Terms. Future J. Pharm. Sci. 2023, 9, 14.
- Liang, Z.; Xu, Y.; Zhang, Y.; Zhang, X.; Song, J.; Jin, J.; Qian, H. Anticancer Applications of Phytochemicals in Gastric Cancer: Effects and Molecular Mechanism. Front. Pharmacol. 2023, 13, 1078090.
- Mandal, M.K.; Mohammad, M.; Parvin, S.I.; Islam, M.M.; Gazi, H.A.R. A Short Review on Anticancer Phytochemicals. Pharmacogn. Rev. 2023, 17, 11–23.
- Curti, V.; Di Lorenzo, A.; Dacrema, M.; Xiao, J.; Nabavi, S.M.; Daglia, M. In Vitro Polyphenol Effects on Apoptosis: An Update of Literature Data. Semin. Cancer Biol. 2017, 46, 119–131.
- Cook, M.T.; Mafuvadze, B.; Besch-Williford, C.; Ellersieck, M.R.; Goyette, S.; Hyder, S.M. Luteolin Suppresses Development of Medroxyprogesterone Acetate-Accelerated 7,12-Dimethylbenz(a)Anthracene-Induced Mammary Tumors in Sprague-Dawley Rats. Oncol. Rep. 2016, 35, 825–832.
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in Cancer Prevention: New Insights (Review). Int. J. Funct. Nutr. 2020, 1, 9.
- Ali, M.; Benfante, V.; Stefano, A.; Yezzi, A.; Di Raimondo, D.; Tuttolomondo, A.; Comelli, A. Anti-Arthritic and Anti-Cancer Activities of Polyphenols: A Review of the Most Recent In Vitro Assays. Life 2023, 13, 361.
- Jurczyk, M.; Kasperczyk, J.; Wrześniok, D.; Beberok, A.; Jelonek, K. Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy. Biomedicines 2022, 10, 1187.
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S.; et al. Resveratrol for Cancer Therapy: Challenges and Future Perspectives. Cancer Lett. 2021, 515, 63–72.
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15.
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447.
- Bozorgi, A.; Haghighi, Z.; Khazaei, M.R.; Bozorgi, M.; Khazaei, M. The Anti-Cancer Effect of Chitosan/Resveratrol Polymeric Nanocomplex against Triple-Negative Breast Cancer; an in Vitro Assessment. IET Nanobiotechnol. 2023, 17, 91–102.
- Muller, A.G.; Sarker, S.D.; Fatokun, A.A.; Hutcheon, G.A. Formulation of Resveratrol into PGA-Co-PDL Nanoparticles Increases Its Cytotoxic Potency against Lung Cancer Cells. RPS Pharm. Pharmacol. Rep. 2023, 2, rqac007.
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626.
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-Targeted Delivery of Resveratrol Using Solid Lipid Nanoparticles Functionalized with Apolipoprotein E. J. Nanobiotechnol. 2016, 14, 27.
- Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Novel Resveratrol and 5-Fluorouracil Coencapsulated in PEGylated Nanoliposomes Improve Chemotherapeutic Efficacy of Combination against Head and Neck Squamous Cell Carcinoma. BioMed Res. Int. 2014, 2014, 424239.
- Khatun, M.; Choudhury, S.; Liu, B.; Lemmens, P.; Pal, S.K.; Mazumder, S. Resveratrol–ZnO Nanohybrid Enhanced Anti-Cancerous Effect in Ovarian Cancer Cells through ROS. RSC Adv. 2016, 6, 105607–105617.
- Aras, A.; Khokhar, A.R.; Qureshi, M.Z.; Silva, M.F.; Sobczak-Kupiec, A.; Pineda, E.A.G.; Hechenleitner, A.A.W.; Farooqi, A.A. Targeting Cancer with Nano-Bullets: Curcumin, EGCG, Resveratrol and Quercetin on Flying Carpets. Asian Pac. J. Cancer Prev. 2014, 15, 3865–3871.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931.
- Mekuye, B.; Abera, B. Nanomaterials: An Overview of Synthesis, Classification, Characterization, and Applications. Nano Sel. 2023, 4, 486–501.
- Singh, V.; Yadav, S.S.; Chauhan, V.; Shukla, S.; Vishnolia, K.K. Applications of Nanoparticles in Various Fields: In Advances in Medical Technologies and Clinical Practice; Yadav, D., Bansal, A., Bhatia, M., Hooda, M., Morato, J., Eds.; IGI Global: Hershey, PA, USA, 2021; pp. 221–236. ISBN 978-1-79986-527-8.
- Kumari, S.; Raturi, S.; Kulshrestha, S.; Chauhan, K.; Dhingra, S.; András, K.; Thu, K.; Khargotra, R.; Singh, T. A Comprehensive Review on Various Techniques Used for Synthesizing Nanoparticles. J. Mater. Res. Technol. 2023, 27, 1739–1763.
- Park, E.-J.; Pezzuto, J.M. The Pharmacology of Resveratrol in Animals and Humans. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1071–1113.
- Tellone, E.; Galtieri, A.; Russo, A.; Giardina, B.; Ficarra, S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2015, 2015, 392169.
- Morales, M.; Barcelo, A.; Pedreño, M. Plant Stilbenes: Recent Advances in Their Chemistry and Biology. Adv. Plant Physiol. 2000, 3, 39–70.
- Bostanghadiri, N.; Pormohammad, A.; Chirani, A.S.; Pouriran, R.; Erfanimanesh, S.; Hashemi, A. Comprehensive Review on the Antimicrobial Potency of the Plant Polyphenol Resveratrol. Biomed. Pharmacother. 2017, 95, 1588–1595.
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91.
- Jojima, K.; Tanaka, A.; Node, K. Resveratrol Supplementation: A Therapeutic Potential for Cardiac Remodeling in Hypertensive Heart Disease. Hypertens. Res. 2023, 46, 1596–1598.
- Chen, X.; Song, X.; Zhao, X.; Zhang, Y.; Wang, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Insights into the Anti-Inflammatory and Antiviral Mechanisms of Resveratrol. Mediat. Inflamm. 2022, 2022, 7138756.
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol Brain Delivery for Neurological Disorders Prevention and Treatment. Front. Pharmacol. 2018, 9, 1261.
- Clouser, C.L.; Chauhan, J.; Bess, M.A.; Van Oploo, J.L.; Zhou, D.; Dimick-Gray, S.; Mansky, L.M.; Patterson, S.E. Anti-HIV-1 Activity of Resveratrol Derivatives and Synergistic Inhibition of HIV-1 by the Combination of Resveratrol and Decitabine. Bioorganic Med. Chem. Lett. 2012, 22, 6642–6646.
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral Activity of Resveratrol against Human and Animal Viruses. Adv. Virol. 2015, 2015, 184241.
- Heredia, A.; Davis, C.; Redfield, R. Synergistic Inhibition of HIV-1 in Activated and Resting Peripheral Blood Mononuclear Cells, Monocyte-Derived Macrophages, and Selected Drug-Resistant Isolates with Nucleoside Analogues Combined with a Natural Product, Resveratrol. JAIDS J. Acquir. Immune Defic. Syndr. 2000, 25, 246–255.
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220.
- Robinson, K.; Mock, C.; Liang, D. Pre-Formulation Studies of Resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469.
- Amri, A.; Chaumeil, J.; Sfar, S.; Charrueau, C. Administration of Resveratrol: What Formulation Solutions to Bioavailability Limitations? J. Control. Release 2012, 158, 182–193.
- Perrone, D.; Fuggetta, M.P.; Ardito, F.; Cottarelli, A.; De Filippis, A.; Ravagnan, G.; De Maria, S.; Lo Muzio, L. Resveratrol (3,5,4′-Trihydroxystilbene) and Its Properties in Oral Diseases. Exp. Ther. Med. 2017, 14, 3–9.
- Pettit, G.R.; Grealish, M.P.; Jung, M.K.; Hamel, E.; Pettit, R.K.; Chapuis, J.-C.; Schmidt, J.M. Antineoplastic Agents. 465. Structural Modification of Resveratrol: Sodium Resverastatin Phosphate. J. Med. Chem. 2002, 45, 2534–2542.
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, Bioactivity, Encapsulation and Structural Modifications. A Review of Their Current Limitations and Promising Approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 7269–7287.
- Jeon, D.; Jo, M.; Lee, Y.; Park, S.-H.; Phan, H.T.L.; Nam, J.H.; Namkung, W. Inhibition of ANO1 by Cis-and Trans-Resveratrol and Their Anticancer Activity in Human Prostate Cancer PC-3 Cells. Int. J. Mol. Sci. 2023, 24, 1186.
- Santos, A.C.; Pereira, I.; Magalhães, M.; Pereira-Silva, M.; Caldas, M.; Ferreira, L.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Targeting Cancer Via Resveratrol-Loaded Nanoparticles Administration: Focusing on In Vivo Evidence. AAPS J. 2019, 21, 57.
- Dariya, B.; Girish, B.P.; Merchant, N.; Srilatha, M.; Nagaraju, G.P. Resveratrol: Biology, Metabolism, and Detrimental Role on the Tumor Microenvironment of Colorectal Cancer. Nutr. Rev. 2023, nuad133.
- Santos, A.C.; Veiga, F.; Ribeiro, A.J. New Delivery Systems to Improve the Bioavailability of Resveratrol. Expert Opin. Drug Deliv. 2011, 8, 973–990.
- Plauth, A.; Geikowski, A.; Cichon, S.; Wowro, S.J.; Liedgens, L.; Rousseau, M.; Weidner, C.; Fuhr, L.; Kliem, M.; Jenkins, G.; et al. Hormetic Shifting of Redox Environment by Pro-Oxidative Resveratrol Protects Cells against Stress. Free Radic. Biol. Med. 2016, 99, 608–622.
- Singh, G.; Pai, R.S. Optimized PLGA Nanoparticle Platform for Orally Dosed Trans-Resveratrol with Enhanced Bioavailability Potential. Expert Opin. Drug Deliv. 2014, 11, 647–659.
- Albuquerque, B.; Costa, M.S.; Peça, I.N.; Cardoso, M.M. Production of Double-walled Nanoparticles Containing Meloxicam. Polym. Eng. Sci. 2013, 53, 146–152.
- Wenzel, E.; Somoza, V. Metabolism and Bioavailability of Trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481.
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382.
- Almeida, T.C.; Melo, A.S.; Lima, A.P.B.; Branquinho, R.T.; da Silva, G.N. Resveratrol Induces the Production of Reactive Oxygen Species, Interferes with the Cell Cycle, and Inhibits the Cell Migration of Bladder Tumour Cells with Different TP53 Status. Nat. Prod. Res. 2023, 37, 3838–3843.
- Yousef, M.; Vlachogiannis, I.; Tsiani, E. Effects of Resveratrol against Lung Cancer: In Vitro and In Vivo Studies. Nutrients 2017, 9, 1231.
- Bishayee, A.; Petit, D.M.; Samtani, K. Angioprevention Is Implicated in Resveratrol Chemoprevention of Experimental Hepatocarcinogenesis. J Carcinog. Mutagen. 2010, 1, 102.
- Bhattacharjee, J.; Kirby, M.; Softic, S.; Miles, L.; Salazar-Gonzalez, R.-M.; Shivakumar, P.; Kohli, R. Hepatic Natural Killer T-Cell and CD8+ T-Cell Signatures in Mice with Nonalcoholic Steatohepatitis: Bhattacharjee, Kirby; et al. Hepatol. Commun. 2017, 1, 299–310.
- Arablou, T.; Aryaeian, N.; Khodaverdi, S.; Kolahdouz-Mohammadi, R.; Moradi, Z.; Rashidi, N.; Delbandi, A.-A. The Effects of Resveratrol on the Expression of VEGF, TGF-β, and MMP-9 in Endometrial Stromal Cells of Women with Endometriosis. Sci. Rep. 2021, 11, 6054.
- Pradhan, R.; Paul, S.; Das, B.; Sinha, S.; Dash, S.R.; Mandal, M.; Kundu, C.N. Resveratrol Nanoparticle Attenuates Metastasis and Angiogenesis by Deregulating Inflammatory Cytokines through Inhibition of CAFs in Oral Cancer by CXCL-12/IL-6-Dependent Pathway. J. Nutr. Biochem. 2023, 113, 109257.
- Gołąbek-Grenda, A.; Kaczmarek, M.; Juzwa, W.; Olejnik, A. Natural Resveratrol Analogs Differentially Target Endometriotic Cells into Apoptosis Pathways. Sci. Rep. 2023, 13, 11468.
- Khayat, M.T.; Zarka, M.A.; El-Telbany, D.; Farag, A.; El-Halawany, A.M.; Kutbi, H.I.; Elkhatib, W.F.; Noreddin, A.M.; Khayyat, A.N.; El-Telbany, R.F.A.; et al. Intensification of Resveratrol Cytotoxicity, pro-Apoptosis, Oxidant Potentials in Human Colorectal Carcinoma HCT-116 Cells Using Zein Nanoparticles. Sci. Rep. 2022, 12, 15235.
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol Induces Cell Cycle Arrest and Apoptosis with Docetaxel in Prostate Cancer Cells via a P53/p21WAF1/CIP1 and p27KIP1 Pathway. Oncotarget 2017, 8, 17216–17228.
- Catania, A.; Barrajón-Catalán, E.; Nicolosi, S.; Cicirata, F.; Micol, V. Immunoliposome Encapsulation Increases Cytotoxic Activity and Selectivity of Curcumin and Resveratrol against HER2 Overexpressing Human Breast Cancer Cells. Breast Cancer Res. Treat. 2013, 141, 55–65.
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8.
- Li, D.; Wang, G.; Jin, G.; Yao, K.; Zhao, Z.; Bie, L.; Guo, Y.; Li, N.; Deng, W.; Chen, X.; et al. Resveratrol Suppresses Colon Cancer Growth by Targeting the AKT/STAT3 Signaling Pathway. Int. J. Mol. Med. 2019, 43, 630–640.
- Ghafouri-Fard, S.; Bahroudi, Z.; Shoorei, H.; Hussen, B.M.; Talebi, S.F.; Baig, S.G.; Taheri, M.; Ayatollahi, S.A. Disease-Associated Regulation of Gene Expression by Resveratrol: Special Focus on the PI3K/AKT Signaling Pathway. Cancer Cell Int. 2022, 22, 298.
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031.
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, Death, and Autophagy in Cancer: NF-κB Turns up Everywhere. Cell Death Dis. 2020, 11, 210.
- Lin, H.-Y.; Tang, H.-Y.; Davis, F.B.; Davis, P.J. Resveratrol and Apoptosis: Resveratrol-Induced Apoptosis. Ann. N. Y. Acad. Sci. 2011, 1215, 79–88.
- Chin, Y.-T.; Hsieh, M.-T.; Yang, S.-H.; Tsai, P.-W.; Wang, S.-H.; Wang, C.-C.; Lee, Y.-S.; Cheng, G.-Y.; HuangFu, W.-C.; London, D.; et al. Anti-Proliferative and Gene Expression Actions of Resveratrol in Breast Cancer Cells in Vitro. Oncotarget 2014, 5, 12891–12907.
- Tang, H.-Y.; Shih, A.; Cao, H.J.; Davis, F.B.; Davis, P.J.; Lin, H.-Y. Resveratrol-Induced Cyclooxygenase-2 Facilitates P53-Dependent Apoptosis in Human Breast Cancer Cells. Mol. Cancer Ther. 2006, 5, 2034–2042.
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589.
- Yang, X.; Jiang, T.; Wang, Y.; Guo, L. The Role and Mechanism of SIRT1 in Resveratrol-Regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019, 9, 18424.
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. npj Precis. Oncol. 2017, 1, 35.
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome Encapsulation of Curcumin and Resveratrol in Combination Reduces Prostate Cancer Incidence in PTEN Knockout Mice. Int. J. Cancer 2009, 125, 1–8.
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-loaded Nanomedicines for Cancer Applications. Cancer Rep. 2021, 4, e1353.
- Sarvari, P.; Sarvari, P. Advances in Nanoparticle-Based Drug Delivery in Cancer Treatment. Glob. Transl. Med. 2023, 2, 0394.
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater. Res. 2019, 23, 20.
- Sharma, A.; Madhunapantula, S.V.; Robertson, G.P. Toxicological Considerations When Creating Nanoparticle-Based Drugs and Drug Delivery Systems. Expert Opin. Drug Metab. Toxicol. 2012, 8, 47–69.
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973.
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (Incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and Health Sciences 1115 Pharmacology and Pharmaceutical Sciences 09 Engineering 0903 Biomedical Engineering Prof Ueli Aebi, Prof Peter Gehr. J. Nanobiotechnol. 2018, 16, 71.
- Khushnud, T.; Mousa, S.A. Potential Role of Naturally Derived Polyphenols and Their Nanotechnology Delivery in Cancer. Mol. Biotechnol. 2013, 55, 78–86.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Jose, S.; Anju, S.S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. In Vivo Pharmacokinetics and Biodistribution of Resveratrol-Loaded Solid Lipid Nanoparticles for Brain Delivery. Int. J. Pharm. 2014, 474, 6–13.
- Xin, Y.; Liu, T.; Yang, C. Development of PLGA-Lipid Nanoparticles with Covalently Conjugated Indocyanine Green as a Versatile Nanoplatform for Tumor-Targeted Imaging and Drug Delivery. Int. J. Nanomed. 2016, 11, 5807–5821.
- Chu, K.S.; Schorzman, A.N.; Finniss, M.C.; Bowerman, C.J.; Peng, L.; Luft, J.C.; Madden, A.J.; Wang, A.Z.; Zamboni, W.C.; DeSimone, J.M. Nanoparticle Drug Loading as a Design Parameter to Improve Docetaxel Pharmacokinetics and Efficacy. Biomaterials 2013, 34, 8424–8429.
- Cavalcante de Freitas, P.G.; Rodrigues Arruda, B.; Araújo Mendes, M.G.; Barroso de Freitas, J.V.; da Silva, M.E.; Sampaio, T.L.; Petrilli, R.; Eloy, J.O. Resveratrol-Loaded Polymeric Nanoparticles: The Effects of D-α-Tocopheryl Polyethylene Glycol 1000 Succinate (TPGS) on Physicochemical and Biological Properties against Breast Cancer In Vitro and In Vivo. Cancers 2023, 15, 2802.
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. IJMS 2019, 20, 1381.
- Paiva-Santos, A.C.; Veiga, F.; Sequeira, J.; Fortuna, A.; Falcão, A.; Pereira, I.; Pattekari, P.; Ribeiro, C.; Ribeiro, A. First-Time Orally Administered Resveratrol-Loaded Layer-by-Layer Nanoparticles to Rats—A Pharmacokinetics Study. Analyst 2019, 144, 2062–2079.
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Increased Oral Bioavailability of Resveratrol by Its Encapsulation in Casein Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816.
- Augustin, M.A.; Sanguansri, L.; Lockett, T. Nano- and Micro-Encapsulated Systems for Enhancing the Delivery of Resveratrol. Ann. N. Y. Acad. Sci. 2013, 1290, 107–112.
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244.
- Vijayakumar, M.R.; Vajanthri, K.Y.; Balavigneswaran, C.K.; Mahto, S.K.; Mishra, N.; Muthu, M.S.; Singh, S. Pharmacokinetics, Biodistribution, in Vitro Cytotoxicity and Biocompatibility of Vitamin E TPGS Coated Trans Resveratrol Liposomes. Colloids Surf. B Biointerfaces 2016, 145, 479–491.
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the Therapeutic Efficacy of Nanoparticles for Cancer Treatment Using Versatile Targeted Strategies. J. Hematol. Oncol. 2022, 15, 132.
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936.
- Amin, M.; Huang, W.; Seynhaeve, A.L.B.; Ten Hagen, T.L.M. Hyperthermia and Temperature-Sensitive Nanomaterials for Spatiotemporal Drug Delivery to Solid Tumors. Pharmaceutics 2020, 12, 1007.
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-Responsive Nanomaterials for Controlled Drug Delivery. Nanoscale 2014, 6, 12273–12286.
- Yang, Y.; Sun, W. Recent Advances in Redox-Responsive Nanoparticles for Combined Cancer Therapy. Nanoscale Adv. 2022, 4, 3504–3516.
- Liu, J.F.; Jang, B.; Issadore, D.; Tsourkas, A. Use of Magnetic Fields and Nanoparticles to Trigger Drug Release and Improve Tumor Targeting. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1571.
- Awad, N.S.; Paul, V.; AlSawaftah, N.M.; Ter Haar, G.; Allen, T.M.; Pitt, W.G.; Husseini, G.A. Ultrasound-Responsive Nanocarriers in Cancer Treatment: A Review. ACS Pharmacol. Transl. Sci. 2021, 4, 589–612.
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Lye Chew, C.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Chin Wei, L. Advanced in Developmental Organic and Inorganic Nanomaterial: A Review. Bioengineered 2020, 11, 328–355.
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-Loaded Nanoparticles Based on Poly(Epsilon-Caprolactone) and Poly(d,l-Lactic-Co-Glycolic Acid)–Poly(Ethylene Glycol) Blend for Prostate Cancer Treatment. Mol. Pharm. 2013, 10, 3871–3881.
- Yao, Q.; Hou, S.-X.; He, W.-L.; Feng, J.-L.; Wang, X.-C.; Fei, H.-X.; Chen, Z.-H. Study on the preparation of resveratrol chitosan nanoparticles with free amino groups on the surface. Zhongguo Zhong Yao Za Zhi 2006, 31, 205–208.
