Resveratrol is a polyphenolic compound that has gained considerable attention in the past decade due to its multifaceted therapeutic potential, including anti-inflammatory and anticancer properties. However, its anticancer efficacy is impeded by low water solubility, dose-limiting toxicity, low bioavailability, and rapid hepatic metabolism. To overcome these hurdles, various nanoparticles such as organic and inorganic nanoparticles, liposomes, polymeric nanoparticles, dendrimers, solid lipid nanoparticles, gold nanoparticles, zinc oxide nanoparticles, zeolitic imidazolate frameworks, carbon nanotubes, bioactive glass nanoparticles, and mesoporous nanoparticles were employed to deliver resveratrol, enhancing its water solubility, bioavailability, and efficacy against various types of cancer.
Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
- anticancer properties
- bioavailability
- nanoparticles
- polyphenolic compound
- resveratrol encapsulation
1. Introduction
Cancer represents a significant global health challenge, standing as the second most common and prevalent cause of mortality. According to the World Health Organization (WHO), 10 million people died in 2020, a figure projected to increase by 70% in the next two decades [1,2]. The estimated worldwide economic cost of cancer is USD 25.2 trillion for 2020 to 2050 [3]. Conventional treatments include chemotherapy, immunotherapy, radiotherapy, and surgery [4,5]. However, modalities such as chemotherapy and radiotherapy encounter challenges in the form of radioresistance and chemoresistance. To overcome these limitations, exploring innovative therapeutic molecules and advancing drug delivery systems is imperative to effectively treat various cancer forms [6,7].
Plant-derived sources present an alternative reservoir of bioactive compounds with potential applications of therapeutic or prophylactic agents against various diseases [8,9,10,11,12,13,14,15]. To date, 350,000 vascular plant species are known, and new species are discovered yearly [16]. It is still a broad and understudied area of research with many prospects for new therapeutic development. However, active substances can be extracted and serve as valuable resources for medicinal applications and as building blocks for synthetic and semi-synthetic substances [17]. Among the diverse array of phytochemicals, encompassing terpenes, alkaloids, essential oils, flavonoids, gums, and a range of primary and secondary metabolic components, discernible medicinal effects have been identified [18,19]. A notable statistic underscores the significance of natural origins, indicating that 51% of the 1211 newly approved small-molecule drugs worldwide between 1981 and 2014 were derived from natural products [20]. Polyphenols have been known to have various preventive effects on different conditions such as diabetes, cardiovascular disease, neurodegenerative disorder, and obesity [21,22,23,24,25,26,27,28,29], and have been extensively studied to determine their anticancer potential and incorporate them into cancer treatment modalities like chemotherapy and targeted therapy [30,31,32,33].
Resveratrol (RSV), a stilbenoid polyphenolic compound, has emerged as a promising anticancer agent. However, its therapeutic potential is hindered due to its pharmacokinetic properties, such as chemical instability (due to oxidation and photosensitivity), low water solubility, low bioavailability, rapid metabolism, and elimination [34,35,36,37]. Many attempts have been made to overcome these hurdles using nanoparticles. The encapsulation of resveratrol in nanoparticles increases its absorption, bioavailability, and sustained release [38,39,40,41,42,43,44]. Cutting-edge nanoparticle technology has revolutionized engineers’ and scientists’ approaches to various fields of study. Nanoparticles are advancing the development of novel drug delivery systems, material engineering, and diagnostic sciences [45,46,47,48].
2. Resveratrol
Resveratrol (RSV) (3,5,4′-trihydroxystilbene) is a well-known naturally occurring polyphenolic compound present in various types of plants such as legumes, blueberries, cranberries, grapes, eucalyptus, and various grasses [49,50]. RSV is a secondary metabolite in different plant families, such as Gnetaceae, Dipterocarpaceae, Leguminosae, and Cyperaceae. Moreover, plants produce RSV in response to pathogen attacks, UV radiation, damage, stress, and exposure to ozone [51]. RSV can be modified into various structures, e.g., pterostilbene, 4,4′-hydroxy-trans-stilbene, monoalkoxy, dialkoxy derivatives, and trans 3,4′,5-trimethoxystilbene [52]. RSV has been confirmed to have many health benefits, such as antiviral, antioxidant, anti-inflammatory, neurological, and heart-disease-prevention properties [53,54,55,56]. RSV also enhances the antiviral activity of various drugs, such as zidovudine, zalcitabine, and didanosine [57,58,59]. The anticancer properties of RSV against multiple malignancies were first described by Jang et al. in 1997 [60].
2.1. The Structure and Physical Properties of Resveratrol
Resveratrol is a hydrophobic compound characterized by a molecular weight of 228.25 g/mol and a melting point of 254 °C [61]. The solubility of RSV is 30 µg/mL in water. RSV is soluble in polar compounds, especially dimethyl sulfoxide (DMSO) and ethanol [61,62]. It exists in two isoforms, i.e., trans-RSV and cis-RSV, depicted in Figure 1. The trans-isomer is more stable and predominant, with more therapeutic properties [63]. Cytotoxicity studies on pancreatic cancer, breast cancer, small-cell lung carcinoma, colon cancer, and prostate cancer cell lines revealed that trans-resveratrol possesses slightly more potent cytotoxic properties than the cis-isomer, attributed to its better bioavailability and biodistribution [64,65,66]. Upon exposure to sunlight or UV radiation at 254 nm or 366 nm, trans-RSV converts into cis-RSV and vice versa. Trans-RSV is more thermo- and photo-stable than cis-RSV. Trans-RSV remains stable in neutral aqueous buffers for 42 h and 28 days at acidic pH; however, cis-RSV remains stable at neutral pH [67,68]. Additionally, RSV becomes unstable when exposed to high humidity and prolonged exposure to light [69]. Additionally, there is evidence that RSV oxidizes into quinines and semiquinones, which cause cell damage [70].
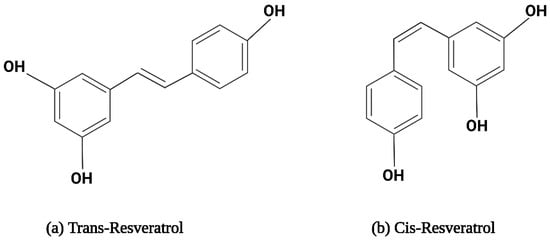
Figure 1. The structural formula for resveratrol isomers.
2.2. Metabolism of Resveratrol
RSV has a half-life of 8 to 14 min in plasma after oral treatment, and its plasma concentrations are often low, sometimes not detectable at all [71]. Upon oral administration, RSV is absorbed by enterocytes, which undergo sulfate conjugation and glucuronidation in the liver and intestine, leading to the formation of trans-resveratrol-3-sulfate and trans-resveratrol-3-O-glucuronide metabolites [36]. Meanwhile, a small quantity of free RSV remains in the blood circulation after being absorbed by plasma proteins like albumin, blood cells, and lipoproteins [72]. Primarily, RSV is administered orally; different levels of free RSV can be detected in urine, ranging from negligible quantities to 17%. The sulfated form of cis-RSV-4′-sulfate is more prevalent than the glucuronidated form. In addition, several studies have found that a minor amount of RSV metabolites are excreted in human feces [73,74].
2.3. Mechanism of Action of Resveratrol against Cancer
RSV is known for its anticancerous properties, mediating apoptosis, cell growth, metastasis, and angiogenesis, as illustrated in Figure 2 [75,76,77,78,79,80,81,82]. Its mechanism involves reducing angiogenesis and increasing apoptosis through the inhibition of vascular endothelial growth factor (VEGF) expression by downregulating hypoxia-inducible factor 1 (HIF-1) [76,77]. RSV promotes apoptosis by arresting the cell cycle at G0/G1 by upregulating the expression of cyclin-dependent kinase (CDK) inhibitors p21 and p27. It also upregulates the expression of cyclin D1, CDK 4, and CDK6 [83].
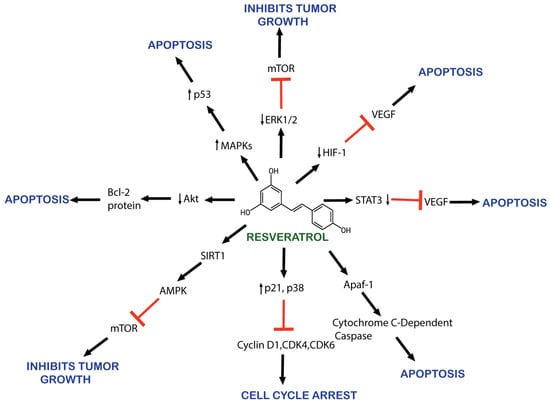
Figure 2. Schematic representation of resveratrol’s anticancer effects.
RSV causes the apoptosis of cancerous cells by downregulating HER2/neu expression [84]. RSV also inhibits cancer-promoting molecular pathways, such as nuclear factor kappa B (NF-kB), PI3K/AKT/mTOR, and STAT3 [85]. AKT serine/threonine kinase is an oncogene protein involved in cell survival, apoptosis, proliferation, and growth. It is involved in the phosphoinositide 3-kinase (PI3K)/AKT signaling pathway activated by inflammation, DNA damage, and growth factors [86]. AKT overexpression has been observed in various cancers [87,88]. RSV also inhibits NF-kB-regulated genes such as VEGF, B cell lymphoma protein-2 (Bcl-2), B-cell lymphoma-extra-large (Bcl-xL), and matrix metalloproteinase (MMP). As NF-kB activates, it alters caspase activity and increases antiapoptotic gene expression, promoting cell proliferation and protecting cells from apoptosis [89].
Moreover, RSV also activates the p53 kinase mediated by MAPKs (mitogen-activated protein kinases) [90]. RSV induces the activation of Apaf-1, which involves activating cytochrome C-dependent caspase and triggers a cascade of apoptosis events [91]. RSV inhibits the activity of the cyclooxygenase enzyme (COX) which converts arachidonic acid to prostaglandin, an inflammatory factor that induces tumor cell proliferation [92,93]. RSV also activates the SIRT1 enzyme, which deacetylates histone and non-histone proteins. SIRT1 regulates inflammation, cell cycle defects, and metabolic control [94,95]. In vitro research demonstrates RSV’s efficacy at reducing cell proliferation and promoting apoptosis by downregulating molecular targets, including p-Akt, cyclin D1, the mammalian target of rapamycin, and androgen receptor (AR) protein [96].
Despite its therapeutic potential, RSV has low gastrointestinal (GI) absorption because of its low water solubility and quick metabolism, and the degradation of RSV by oxidative enzymes [97]. Moreover, cancer cells can develop resistance to chemotherapeutic drugs due to mutations known as multidrug resistance (MDR). Other carcinogenesis processes lead to MDR, including pathways leading to apoptosis, DNA damage response, downstream signaling pathways, changes in drug efflux attributable to modifications in proteins involved in drug transfer from the cell membrane, changes in enzymes involved in drug processing and metabolism, changes in the composition of the cell membrane, cancer stem cells (CSCs), epithelial–mesenchymal transition, and changes in the tumor environment [98].
A potential solution to these problems involves the development of nanoparticles capable of carrying resveratrol, ensuring targeted delivery without inducing toxicity [97].
Critical considerations in nanoparticle design, crucial for an effective drug delivery system, include the following:
3. Application of Nanoparticles to Improve the Therapeutic Potential of Resveratrol for Cancer
In nanotechnology, a particle is often categorized according to its physical diameter: ultrafine particles typically have a physical diameter between 1 and 100 nm in at least one dimension [40]. Drugs can be conjugated to nanoparticle surfaces through covalent or ionic bonds, structural absorption, or encapsulation inside nanoparticle cores [103]. These nanoparticle-based formulations can increase absorption, bioavailability, and chemical integrity, enhance permeability and retention effect (EPR) across the biological membrane, and ensure the optimal dosage of drugs reaches the cancer target cells [104,105]. Biodistribution studies have demonstrated that RSV-loaded nanoparticles or RSV-conjugated nanoparticles have a much longer circulation time than free RSV [106,107,108,109]. Nanoparticles protect RSV from rapid metabolism and elimination, resulting in sustained blood levels. According to pharmacokinetic studies, nanoencapsulated RSV has an extended half-life [110]. Pharmacokinetic studies have also revealed that RSV bioavailability is increased when delivered in nanoformulations. Nanoparticles improve RSV absorption in the gastrointestinal tract and protect first-pass metabolism in the liver [111,112,113]. Biodistribution studies revealed that nanoparticles enhanced RSV accumulation in tumor tissues compared to free RSV due to enhanced permeability and retention (EPR) in tumor vasculature. Nanoformulations provide controlled and sustained RSV release. The controlled release of RSV contributes to prolonged therapeutic effects and decreases the need for frequent dosing [114,115].
Various strategies, e.g., triggered drug release and stimuli-responsive approaches, have been used in RSV nanoformulation to release RSV in response to specific internal or external triggers. These strategies aim to increase therapeutic efficacy and precise control, and minimize the side effects of drug release. The common stimuli-responsive approaches are pH-responsive release, enzyme-responsive release, temperature-responsive release, redox-responsive release, light-responsive release, magnetic-responsive release, and ultrasound-responsive release [116].
In pH-sensitive nanoparticles, RSV is released in response to acidic pH conditions in tumor microenvironments. This pH-triggered release enhances drug delivery to cancer cells while minimizing release in normal tissues [117]. Thermosensitive nanoparticles loaded with RSV release the drug in response to local temperature changes. Hyperthermia treatment can trigger drug release [118]. The enzyme-triggered release of RSV can be achieved by conjugating enzyme-cleavable compounds in the nanoparticle structure. The presence of disease-specific enzymes, such as matrix metalloproteinases in tumors, releases RSV [119]. Redox-responsive nanoparticles release RSV in response to elevated reactive oxygen species (ROS) at the tumor site. Incorporating photo-responsive materials into resveratrol nanoparticles allows light-induced drug release [120]. Resveratrol-loaded magnetic nanoparticles can be guided to specific target sites using external magnets. Magnetic fields induce drug release at the desired location [121]. Ultrasound-responsive nanoparticles loaded with resveratrol can be triggered to release the drug at the target site using focused ultrasound waves, providing spatial and temporal control. These stimuli-activated approaches offer precise control over drug-release kinetics, improving resveratrol’s therapeutic index and minimizing systemic side effects. The trigger mechanism’s choice depends on the target tissue’s specific characteristics and the desired therapeutic outcome [122].
Nanoparticles can broadly be categorized into organic and inorganic types, with recent extensive studies focusing on organic particles. Specifically, liposomes, polymersomes, polymer constructions, and micelles are used for imaging and drug and gene delivery methods. Although inorganic nanoparticles exhibit highly material- and size-dependent physicochemical characteristics, incomparable with conventional lipid- or polymer-based NPs, they have also attracted researchers’ interest in recent years [123]. Various nanoformulations, including liposomes, metallic nanoparticles, solid lipid nanoparticles, micelles, polymeric nanoparticles, and inorganic nanoparticles, are illustrated in Figure 3 and Figure 4.
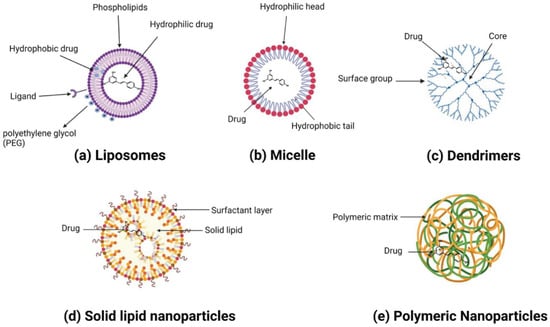
Figure 3. A variety of resveratrol-loaded organic nanoparticles.
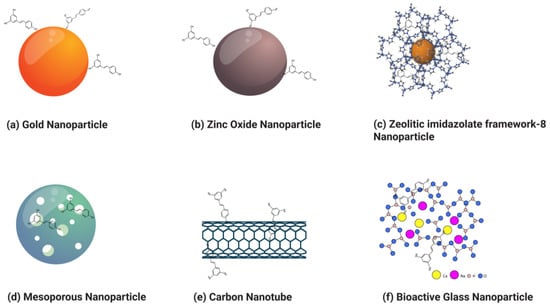
Figure 4. Various types of resveratrol-loaded/conjugated inorganic nanoparticles.
Several attempts have been made to develop nanotechnology-based strategies and increase bioavailability and effectiveness in various cancer models [40,41,42,43,44,124]. In an initial attempt at encapsulating RSV, chitosan NPs were used. The study conducted by Yao et al. showed that sustained release of RSV, with resveratrol-loaded nanoparticles at lower concentrations, caused an increased percentage of cell death compared to an equivalent dose of free resveratrol [125].
This entry is adapted from the peer-reviewed paper 10.3390/ph17010126
This entry is offline, you can click here to edit this entry!
