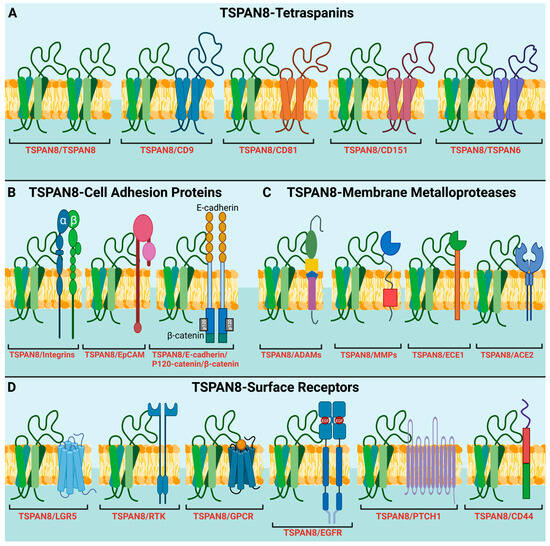
To date, no natural ligands and catalytic activities for TSPAN8 and other tetraspanins have been identified. However, TSPAN8 is known to form a complex with a diverse range of transmembrane receptors in various pathways and regulate their activities. In this section, we mainly discuss the role of TSPAN8 in the Wnt/β-catenin, EGFR, mTOR and hedgehog pathways (Figure 2).
TSPAN8 plays multifaced roles in the Wnt/β-catenin-signaling pathway. In the absence of Wnt signaling activation, β-catenin binds to E-cadherin in the plasma membrane. Upon Wnt signaling activation, β-catenin is translocated to the nucleus, where it functions as a transcriptional cofactor. A study on colorectal cells showed that TSPAN8 directly interacted with β-catenin and enhanced β-catenin expression, which then bound to the TSPAN8 promoter and promoted TSPAN8 transcription [
56]. Similarly, TSPAN8 expression was reported to stabilize β-catenin, which in turn enhanced TSPAN8 transcription in melanoma cells [
57]. Of note is that both studies did not address the specific cellular location of the interaction between β-catenin and TSPAN8. TSPAN8 might also regulate β-catenin transcription by binding to NOTCH2, a receptor of the Notch signaling pathway [
58]. We recently showed that TSPAN8 interacts with LGR5 (leucine-rich repeat-containing G-protein coupled receptor 5), a G protein-coupled receptor involved in the Wnt signaling pathway, to maintain the quiescence of mammary stem cells [
59].
The epidermal growth factor receptor (EGFR), which plays a crucial role in many key cellular processes such as cell proliferation, survival, motility, and differentiation [
60], has been identified as a strong TSPAN8-binding partner [
47]. Several studies have shown that EGF and TSPAN8 expressions are linked in a concentration- and time-dependent manner. For instance, TSPAN8 knockdown attenuated the effects of EGF on gastric cancer cell proliferation and invasion [
61], while the suppression of endogenous EGF expression by KDM2A, a histone demethylase, decreased TSPAN8 expression in breast cancer cells [
62]. Furthermore, SOX9 was identified as a key transcriptional regulator of TSPAN8 expression in response to EGF stimulation in pancreatic cancer cells. The EGF-SOX9-TSPAN8-signaling cascade was shown to regulate cancer cell invasion and metastasis [
63]. Interestingly, CD9 and CD82 have also been found to form a complex with EGFR at the cell surface [
64,
65].
Multiple independent studies investigating single nucleotide polymorphisms (SNPs) and copy number variants (CNVs) have unmasked an association between the genomic locus containing
LGR5/
TSPAN8 and an increased risk of type 2 diabetes in different patient cohorts [
67,
68,
69,
70,
71]. However, the significance of this association needs to be validated in additional studies. Notably, TSPAN8 knockout mouse models did not display apparent alterations in fasting insulin and glucose levels [
70]. Nevertheless, TSPAN8 expression levels were found to be significantly reduced in the blood samples of diabetic nephropathy (DN) patients [
72]. miR-543 was identified as a key regulator for TSPAN8 downregulation in kidney tissues of DN mice [
73]. In an in vitro cell culture system, TSPAN8 was found to form a complex with Rictor (rapamycin-insensitive companion of mammalian target of rapamycin), a crucial component of the mammalian target of rapamycin complex 2 (mTORC2), to regulate high glucose-induced autophagy [
72]. Interestingly, TSPAN8 also formed a complex with integrin α3 and Rictor in glioma cells [
74]. This complex appeared to be necessary for the activation of mTORC2, as TSPAN8 knockdown prevented the assembly of mTOR-Rictor and the downstream phosphorylation of AKT and PKCα.
3. TSPAN8 as a Genetic Marker and Key Regulator of Normal Tissue Stem Cells and Cancer Stem Cells
The expression of
TSPAN8 mRNA is restricted to defined cell lineages, and TSPAN8 has emerged as a key marker and regulator of stem/progenitor cells in different tissues. The researchers previously identified TSPAN8 and LGR5 as molecular markers of quiescent mammary stem cells [
143] and unraveled that TSPAN8 physically interacts with LGR5 to form a functional complex in these cells to regulate their quiescent status (
Figure 2D) [
59,
144]. Interestingly, TSPAN8 expression also defines the progenitor subset in the luminal population of mouse mammary glands [
143]. A recent study reported that
BRCA2 mutation-associated breast cancers potentially originate from TSPAN8
+ luminal progenitor cells [
145]. The suppression of
TSPAN8 expression by the transcriptional factor FOXP1 is essential for quiescent mammary stem cells to re-enter cell cycle for ductal morphogenesis [
59,
144]. A knockout of
FOXP1 led to the constitutive expression of TSPAN8 and prevented the exit of mammary stem cells from quiescence, profoundly blocking the mammary gland development in mice. TSPAN8 has also been identified as a specific marker for spermatogonia stem cells [
146]. In rats, TSPAN8 down-regulation in Sertoli cells plays an obligatory role in the division and differentiation of male germ cells into sperms during puberty. Remarkably, sperm production, in one study, was almost completely blocked in a transgenic rat model where TSPAN8 downregulation in Sertoli cells was prevented from puberty up to adulthood [
147].
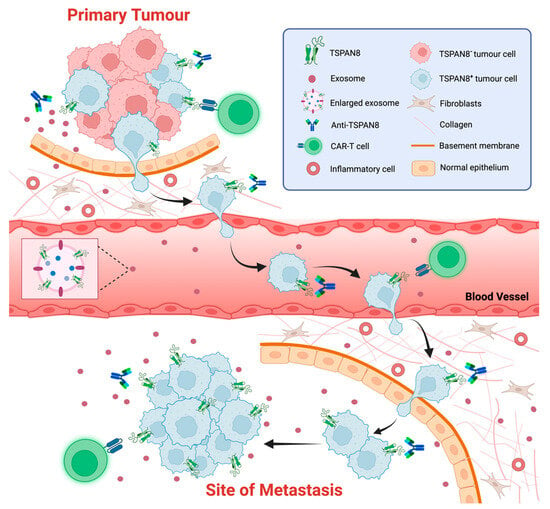
TSPAN8 expression also marks cancer stem cells (CSCs) or cancer-initiating cells (CICs) in various cancer types (
Figure 4). For instance, TSPAN8 is one of the markers used to characterize pancreatic cancer stem cells (Pa-CSCs) [
100]. In another study, TSPAN8, alongside CD44v6, an α6β4 integrin, and CD133, were found to be upregulated in pancreatic cancer-initiating cells (Pa-CIC), where they conferred in vivo growth and metastatic advantages [
148]. TSPAN8 expression was elevated in breast CSCs, where high levels of TSPAN8 conferred on CSCs resistance to chemotherapy [
66]. Mechanistically, TSPAN8 was shown to promote cancer cell stemness in breast cancer through the activation of the sonic Hedgehog (SHH) signaling pathway, which led to the upregulation of stemness-related genes [
66]. Similarly, a positive regulatory loop between β-catenin and TSPAN8 regulated the expression of stemness genes for the maintenance of cancer stemness and the sphere-forming ability of colorectal cancer cells [
56].
Figure 4. TSPAN8 as a biomarker and therapeutic target of cancer. The schematic representation of strategies targeting TSPAN8 at different stages of tumor progression. TSAPN8 expression has been shown to be a molecular marker for cancer stem cells (CSCs) or cancer-initiating cells (CICs). Multiple monoclonal anti-TSPAN8 monoclonal antibodies have been shown to impair cancer progression. They may restrain primary tumor growth and block the migration/invasion of cancer cells, which in turn prevents metastasis. Anti-TSPAN8 antibodies may also suppress the formation of tumor-promoting microenvironments by inhibiting the biogenesis and release of cancer cell-derived exosomes. Additionally, CAR-T cells targeting TSPAN8 may be a novel promising strategy for treatment of various epithelial cancers with TSPAN8 expression. Moreover, detection of TSPAN8-expressing circulating tumor cells or exosomes potentially represents a non-invasive approach for diagnosis and prognosis of various epithelial cancers. Created with BioRender.com, accessed on 16 January 2024.
4. Regulation of TSPAN8 Transcription
Given that TSPAN8 is upregulated and partakes in the progression of various cancers, it is crucial to understand the molecular regulation of
TSPAN8 transcription. AEG-1 (astrocyte elevated gene-1), which promotes tumor progression and metastasis, upregulates
TSPAN8 transcription through the activation of the MEK/ERK signaling pathway [
122]. β-catenin directly promotes
TSPAN8 transcription through a positive feedback loop [
56,
57]. The long non-coding RNA (lncRNA) SOX21 antisense RNA1 (SOX21-AS1), which is overexpressed in several cancers including HCC, colorectal cancers, and lung adenocarcinoma [
149,
150,
151], acts as a positive co-regulator to promote
TSPAN8 transcription by the transcription factor GATA6, therefore increasing the migration and invasion of cancer cells [
152]. In response to EGF stimulation, the transcription factor SOX9 activates
TSPAN8 expression and promotes cell invasion [
63]. In a study using large scale RNAi screens, several genes, including GSK3β, PTEN, IQGAP1, TPT1 and LCMR1, were identified to inhibit or enhance
TSPAN8 transcription. In particular, LCMR1 (lung cancer metastasis-related protein 1) enhanced
TSPAN8 expression and promoted the invasion of melanoma cells [
112].
5. Novel Functions of TSPAN8 in the Nucleus
As described above, tetraspanins are transmembrane proteins that mainly localize and function in the plasma membrane. Studies have shown that TSPAN8 can release from the plasma membrane and translocate to the nucleus in multiple cancer cells. This process seems to be assisted by TSPAN8 palmitoylation and cholesterol binding [
154]. Moreover, the phosphorylation of TSPAN8 by AKT enables the binding of TSPAN8 to 14-3-3θ and importin ß1, which is essential for its nuclear translocation in response to the activation of EGF-EGFR signaling. Nuclear TSPAN8 interacts with the transcription factor STAT3 to activate the transcription of cancer-promoting genes including MYC, BCL-2 and MMP9, which in turn promotes tumorigenesis [
39,
155]. Interestingly, a new humanized monoclonal antibody targeting the LEL domain of TSPAN8 was able to suppress the release of TSPAN8 from the plasma membrane and its subsequent nuclear translocation. Furthermore, androgen was also found to induce a nuclear localization of TSPAN8, resulting in the formation of TSPAN8 and an androgen receptor (AR) complex in prostate cancer cells [
156]. Nuclear TSPAN8 is required for the transcriptional functions of AR and AR variant 7 (AR-V7) [
157]. Of note is that the detailed molecular process for the translocation of a plasma membrane-integrated protein to the nucleus remains unclear. However, the accumulating evidence suggests that distinct plasma membrane proteins, including cell signaling receptors and cell adhesion proteins, are able to move to the nucleus to execute a non-canonical function under certain circumstances [
158,
159].
6. TSPAN8 as a Biomarker and Therapeutic Target of Cancer
TSPAN8 was originally identified as a human tumor-associated antigen found to be overexpressed in many different epithelial cancers, including lung, liver, gastric, esophageal, colorectal, and ovarian carcinomas [
130,
160,
161]. Accumulating evidence suggests that TSPAN8 plays an oncogenic role in the initiation, progression, and metastasis of different epithelial cancers. In line with this, there is a positive correlation between high TSPAN8 expression and clinicopathological characteristics of an aggressive tumor, including tumor differentiation, invasion depth, lymph node metastasis, and clinical stage [
162,
163,
164,
165,
166]. However, there is also some evidence against this [
167,
168].
The potential of employing TSPAN8 mRNA and proteins as novel blood biomarkers for different epithelial cancers has been explored. Based on a systematic large-scale meta-analysis on the blood samples, a panel of mRNAs of four genes—TSPAN8, LGALS4, COL1A2, and CEACAM6—were identified as putative markers of colorectal cancer [
169,
170,
171]. Genome-wide microarray analysis identified
TSPAN8 as a gene that is significantly upregulated in gastric cancers compared to normal gastric mucosae, suggesting its use as a novel molecular marker for the detection of circulating gastric cancer cells in the peripheral blood [
172]. Another study using proteomic analysis showed the selective enrichment of the TSPAN8 protein in EVs from metastatic NSCLC (non-small cell lung cancer) cell lines. Consistent with this, serum EVs from patients with stage III premetastatic NSCLC tumors displayed high TSPAN8 levels. This study highlights the potential of testing TSPAN8 protein levels in EVs from blood for prognosing the metastasis of NSCLC patients [
96]. Collectively, TSPAN8 may represent a promising candidate for use as blood-based biomarkers for cancer screening.
Therapeutic monoclonal antibodies (mAbs) targeting different tetraspanin members including TSPAN8, CD9, CD37 and CD151 have been explored in preclinical models and clinical trials for the treatment of hematological malignancies and carcinomas [
173,
174,
175,
176,
177]. Several monoclonal antibodies against TSPAN8 have been developed and showed a significant inhibition of tumor growth and metastasis in preclinical cancer models. For example, the monoclonal antibody Ts29.2 that specifically targets human TSPAN8 showed significant efficacy in pre-clinical models of CRC [
178]. Similarly, angiogenesis induced by TSPAN8 in rat tumor models could be effectively inhibited by the anti-rat TSPAN8 specific antibody D61.A [
179]. Interestingly, the antibodies were highly selective in inhibiting sprouting endothelial cells and were effective regardless of whether the tumor cells themselves expressed TSPAN8 or not. In recent years, several monoclonal TSPAN8 antibodies specifically targeting the LEL of human TSPAN8 have been developed and tested for their potential in treating different solid tumors. For instance, the humanized monoclonal antibody hT8Ab4 showed an anti-tumor effect in multiple cancers associated with the nuclear translocation of TSPAN8 [
39]. Another humanized monoclonal antibody, C4 scFv-Fc, displayed high affinity binding, even at sub-nanomolar concentrations, to amino acids 140-205 within TSPAN8 LEL in a conformation-dependent manner [
180]. This antibody also significantly reduced the invasion of metastatic colorectal cancer (mCRC) cell lines that express TSPAN8 compared to non-mCRC cell lines. Furthermore, the monoclonal antibody TSPAN8–LEL IgG was shown to significantly reduce the incidence of epithelial ovarian cancer metastasis in vivo without causing severe toxicity [
177]. The exact mechanisms for the tumor-inhibitory effects of these antibodies are yet to be elucidated. The binding of these antibodies to cell-surface TSPAN8 may lead to alternations in TSPAN8-enriched TEMs and subsequent cell signaling cascades.
The cell-surface expression of TSPAN8 can also be used to target and destroy cancer cells, such as through antibody–drug conjugates or CAR-T (chimeric antigen receptor T-cells). In mouse CRC xenograft models, the radiolabeled antibody Ts29.2 demonstrated high specificity in localizing to tumors as well as high efficacy—up to 70%—in reducing tumor burdens [
181]. CAR-T therapy is a ground-breaking immunotherapy for certain types of cancer. However, the identification of suitable tumor-specific antigens for this therapy remains challenging. To discover effective target candidates for pancreatic ductal adenocarcinoma, a strategy combining flow cytometry screenings, bioinformatic expression analyses, and a cyclic immunofluorescence platform was developed. CLA, CD66c, CD318 and TSPAN8 were identified as putative target candidates from 371 tumor antigens. Remarkably, CAR-T cells specifically targeting TSPAN8 showed high efficacy, with complete tumor eradication in some cases. These findings highlight TSPAN8 as a promising candidate, with high potential for successful clinical translation [
182]. Another in vitro study aimed at developing a novel spacer for CAR-T cells to improve their functionality against membrane-proximal epitopes demonstrated efficient engagement of TSPAN8-specific CAR-T cells. Remarkably, the transplantation of these cells in vivo showed excellent tumor-killing efficacy [
183].