Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Thoracoscopic surgical biopsy has shown excellent histological characterization of undetermined interstitial lung diseases, although the morbidity rates reported are not negligible. In delicate patients with interstitial lung disease and restrictive ventilatory impairment, morbidity is thought to be due at least in part to tracheal intubation with single-lung mechanical ventilation; therefore, spontaneous ventilation thoracoscopic lung biopsy (SVTLB) has been proposed as a potentially less invasive surgical option.
- interstitial lung disease
- surgical biopsy
- non-intubated thoracic surgery
- awake thoracic surgery
1. Introduction
Interstitial lung diseases (ILDs) are defined as a heterogeneous group of idiopathic or secondary conditions that share a similar clinical, radiological, and histologic pattern characterized by inflammation and fibrosis involving the lung interstitium [1]. Overall, the prevalence of ILDs is approximately 200 individuals per 100,000 [2]. An early and accurate differential diagnosis among undetermined ILDs is usually pivotal to predicting prognosis and determining the best pharmacological therapy. International consensus guidelines recommend the need for a precise histopathological diagnosis in undetermined ILD patients in whom clinical, laboratory, and high-resolution computed tomography findings, preferably discussed within a multidisciplinary panel, prove insufficient to make a confident diagnosis [2][3]. In these instances, surgical lung biopsy is still the recommended diagnostic procedure despite the proposal of alternative techniques such as transbronchial lung cryobiopsy [4]. Nowadays, mechanical ventilation thoracoscopic lung biopsy (MVTLB), performed under general anesthesia with double-lumen tube intubation, is the preferred surgical diagnostic tool, offering excellent diagnostic yields of 93–95% [5][6]. However, given the typical impaired pulmonary function of ILD patients, the risk of post-operative complications of MVTLB is still not negligible, with reported mortality and morbidity rates of 1.5–2.4% and approximately 16%, respectively [6][7][8][9]. In addition, general anesthesia and single-lung mechanical ventilation entail multiple potential side effects, which are thought to be a risk factor for the acute exacerbation of ILD [7]. In an attempt to combine the excellent diagnostic yield of MVTLB with lower operative risks, spontaneous ventilation thoracoscopic lung biopsy (SVTLB), performed under regional anesthesia without tracheal intubation and single-lung mechanical ventilation, has been proposed as a less invasive surgical alternative [10].
2. Anesthesia and Surgery
Nine studies [10][11][12][13][14][15][16][17][18] reported details about anesthesia. With regard to regional anesthesia, thoracic epidural analgesia was the chosen technique in three studies [11][14][17], while in the other two investigations [13][18], the enrolled population all received intercostal blocks. In the other studies, both procedures were alternatively performed.
Neuromuscular blockers were not employed in any study. When applied, sedation was obtained with propofol and/or remifentanil [10][15][16][18][19], propofol and sufentanil [20], remifentanil or dexmedetomidine [11], or midazolam and fenatanil [14]. One study reported that all patients were fully conscious during the procedure [21], while the others did not mention details about sedation [12][13][17][22].
The surgical approach was mentioned in 9/13 studies [10][11][12][14][17][18][19][20][22]. Out of a total of 360 patients, 257 (71.4%) underwent uniportal VATS while 79 (21.9%) underwent multiportal VATS. One multicentric study [12] also reported 24 surgeries (6.7%) performed using a mini-thoracotomy approach under video assistance. Twelve out of thirteen studies reported a mean number of approximately two biopsies for each operation, usually performed through mechanical stapled wedge resections. In one study from Zhang et al. [22], biopsy forceps were employed to obtain the specimens. Conversion to general anesthesia was reported in 5/675 patients (0.7%). Moreover, conversion to thoracotomy occurred in 2/675 cases (0.3%). At the end of surgery, one chest tube was routinely placed with the exception of two studies [19][20], in which a tubeless approach was carried out. Mean or median operative time was reported in 12 studies [10][11][12][14][15][16][18][19][20][21][22] and ranged from a minimum of 22 min to a maximum of 57.7 min.
3. Histological Results and Diagnostic Yield
The diagnostic yield of surgical biopsies was reported in 12/13 articles. Researchers excluded the study of Hajjar et al. [13] due to a possible selection bias since the authors stated in the material and methods section that all patients had idiopathic pulmonary fibrosis and did not specify if the presence of other diagnoses or undetermined ILD in the histological report was an exclusion criterion.
A final diagnosis was obtained in 631/649 patients (97.2%). Diagnostic yield ranged from 84.6% to 100% (median diagnostic yield of 100%). In seven studies [13][15][16][21][22][23][24], the enrolled population all received a final diagnosis. The paper with the lowest rate was the one by Zhang et al. [22], in which the biopsies were taken with forceps instead of wedge resections.
Ten papers [10][11][12][14][16][17][18][20][21][22] mentioned in detail all histological results. These are summarized in Figure 1.
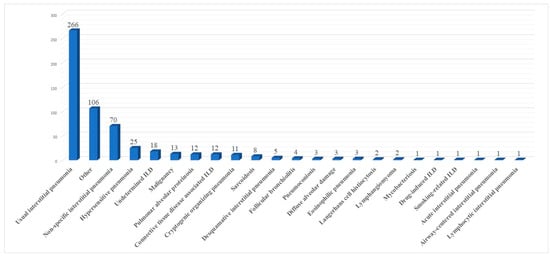
Figure 1. Summary of histological results.
4. Post-Operative Outcome
All the papers included in the review reported data about post-operative morbidity. Out of a total of 675 patients, 64 (9.5%) had a complication, with morbidity rates ranging from 0.0% [11][14] to 27.3% [17] (median morbidity rate of 7.0%). The complications were usually minor: prolonged air leaks in 15/64 patients (23.4%), pneumonia in 5/64 patients (7.8%), atelectasis in 4/64 patients (6.2%), pain at the tube insertion site in 3/64 cases (4.6%), subcutaneous emphysema in 2/64 cases (3.1%), atrial fibrillation, acute exacerbation of ILD, anemia, gastric bleeding, hematoma requiring revision surgery, thromboembolism, pleural effusion and post-operative tube insertion each occurred in 1/64 patients (1.6%), and other non-specified complications in 27/64 cases (42.1%), 18 of which belonged to Clavien-Dindo grade I [23]. There was no 30-day mortality in the reviewed articles. When reported, hospitalization ranged from a minimum mean value of 1 day [14] to a maximum median value of 8 days [17].
5. Comparison between SVTLB and MVTLB
Six articles compared SVTLB to MVTLB for ILD [11][13][14][15][18][21]. A total of 178 patients belonged to the former group while 289 patients belonged to the latter. Post-operative complications were recorded in 13/178 patients (7.3%) in the SVTLB group (median morbidity rate of 5.2%) and 71/289 patients (24.6%) in the other group (median morbidity rate of 24.0%). The meta-analysis showed a significantly lower risk of complications (p < 0.001) in patients submitted to SVTLB, with an Odds Ratio (OR) of 0.31 (95% confidence interval 0.16–0.59). The studies were homogeneous as I2 = 0.0% (Figure 2). With reference to the other main outcome of the review, the comparison of diagnostic yield was performed with five studies. The study of Hajjar et al. [13] was excluded due to selection bias. In addition, no meta-analysis was performed for the diagnostic yield outcome measure since a final diagnosis was reached in all but one case in the MVTLB group [18].
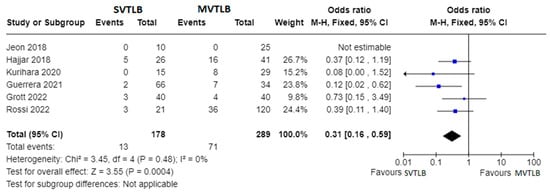
Figure 2. Forest plots of morbidity rate. Squares represent point estimates of the morbidity rate from each study reporting results eligible for analysis. Solid lines represent a 95% confidence interval (CI). The diamond represents the overall pooled effect from the included studies [11][13][14][15][18][21].
Apart from primary outcomes, there was no difference in mortality (Figure 3). Data were not homogeneous and complete enough to perform a meta-analysis for operative time, anesthesia time, and length of hospital stay. However, three investigations reported significantly shorter mean operative times for the SVTLB group: 32.5 ± 18.5 min versus 50.8 ± 18.4 min (p = 0.004) in the study by Kurihara et al. [14]; 38 min versus 77 min (p < 0.001) in the study by Guerrera et al. [15]; 27.4 ± 14.4 min versus 36.4 ± 15.1 min (p = 0.008) in the study by Grott et al. [18]. Conversely, Jeon et al. [11] reported no significant difference (p = 0.25) in operative time, although they found a median value of anesthesia time of 66 min (IQR 62–82) for SVTLB versus 83 min (IQR 69–99) in the MVTLB group (p = 0.025). On the other hand, Grott et al. [18] reported a significantly higher anesthesia induction time for the SVTLB group (24.1 ± 15.6 versus 13.9 ± 9.7 min, p < 0.001). With reference to the length of hospital stay, significantly shorter times in the SVTLB group were reported by Kurihara et al. [14] (1.0 ± 1.3 versus 10.0 ± 34.7 days, p < 0.001), Guerrera et al. [15] (3.1 versus 6.7 days, p = 0.0002), and Grott et al. [18] (3.8 ± 1.6 versus 5.7 ± 2.0 days, p < 0.001).
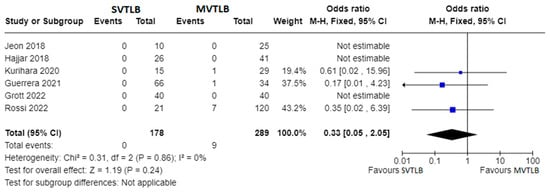
Figure 3. Forest plots of mortality rate. Squares represent point estimates of mortality rate from each study reporting results eligible for analysis. Solid lines represent a 95% confidence interval (CI). The diamond represents the overall pooled effect from the included studies [11][13][14][15][18][21].
This entry is adapted from the peer-reviewed paper 10.3390/jcm13020374
References
- George, P.M.; Spagnolo, P.; Kreuter, M.; Altinisik, G.; Bonifazi, M.; Martinez, F.J.; Molyneaux, P.L.; Renzoni, E.A.; Richeldi, L.; Tomassetti, S.; et al. Progressive fibrosing interstitial lung disease: Clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir. Med. 2020, 8, 925–934.
- Sharp, M.; Mustafa, A.M.; Farah, N.; Bonham, C.A. Interstitial Lung Disease and Sarcoidosis. Clin. Chest Med. 2023, 44, 575–584.
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47.
- Lentz, R.J.; Argento, A.C.; Colby, T.V.; Rickman, O.B.; Maldonado, F. Transbronchial cryobiopsy for diffuse parenchymal lung disease: A state-of-the-art review of procedural techniques, current evidence, and future challenges. J. Thorac. Dis. 2017, 9, 2186–2203.
- Iftikhar, I.H.; Alghothani, L.; Sardi, A.; Berkowitz, D.; Musani, A.I. Transbronchial Lung Cryobiopsy and Video-assisted Thoracoscopic Lung Biopsy in the Diagnosis of Diffuse Parenchymal Lung Disease. A Meta-analysis of Diagnostic Test Accuracy. Ann. Am. Thorac. Soc. 2017, 14, 1197–1211.
- Han, Q.; Luo, Q.; Xie, J.X.; Wu, L.L.; Liao, L.Y.; Zhang, X.X.; Chen, R.C. Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2015, 149, 1394–1401.e1.
- Durheim, M.T.; Kim, S.; Gulack, B.C.; Burfeind, W.R.; Gaissert, H.A.; Kosinski, A.S.; Hartwig, M.G. Mortality and Respiratory Failure After Thoracoscopic Lung Biopsy for Interstitial Lung Disease. Ann. Thorac. Surg. 2017, 104, 465–470.
- Sigurdsson, M.I.; Isaksson, H.J.; Gudmundsson, G.; Gudbjartsson, T. Diagnostic surgical lung biopsies for suspected interstitial lung diseases: A retrospective study. Ann. Thorac. Surg. 2009, 88, 227–232.
- Hutchinson, J.P.; McKeever, T.M.; Fogarty, A.W.; Navaratnam, V.; Hubbard, R.B. Surgical lung biopsy for the diagnosis of interstitial lung disease in England: 1997–2008. Eur. Respir. J. 2016, 48, 1453–1461.
- Pompeo, E.; Rogliani, P.; Cristino, B.; Schillaci, O.; Novelli, G.; Saltini, C. Awake thoracoscopic biopsy of interstitial lung disease. Ann. Thorac. Surg. 2013, 95, 445–452.
- Jeon, C.S.; Yoon, D.W.; Moon, S.M.; Shin, S.; Cho, J.H.; Lee, S.M.; Ahn, H.J.; Kim, J.A.; Yang, M. Non-intubated video-assisted thoracoscopic lung biopsy for interstitial lung disease: A single-center experience. J. Thorac. Dis. 2018, 10, 3262–3268.
- Pompeo, E.; Rogliani, P.; Atinkaya, C.; Guerrera, F.; Ruffini, E.; Iñiguez-Garcia, M.A.; Peer, M.; Voltolini, L.; Caviezel, C.; Weder, W.; et al. Nonintubated surgical biopsy of undetermined interstitial lung disease: A multicentre outcome analysis. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 744–750.
- Hajjar, W.M.; Al-Nassar, S.A.; Al-Sugair, G.S.; Al-Oqail, A.; Al-Mansour, S.; Al-Haweel, R.; Hajjar, A.W. Evaluation of safety and efficacy of regional anesthesia compared with general anesthesia in thoracoscopic lung biopsy procedure on patient with idiopathic pulmonary fibrosis. Saudi J. Anaesth. 2018, 12, 46–51.
- Kurihara, C.; Tolly, B.; DeWolf, A.; Nader, A.; Kim, S.; Odell, D.D.; Argento, A.C.; Budinger, G.R.S.; Bharat, A. Thoracoscopic lung biopsy under regional anesthesia for interstitial lung disease. Reg. Anesth. Pain. Med. 2020, 45, 255–259.
- Guerrera, F.; Costardi, L.; Rosboch, G.L.; Lyberis, P.; Ceraolo, E.; Solidoro, P.; Filippini, C.; Verri, G.; Brazzi, L.; Albera, C.; et al. Awake or intubated surgery in diagnosis of interstitial lung diseases? A prospective study. ERJ Open Res. 2021, 7, 00630–02020.
- Cherchi, R.; Ferrari, P.A.; Guerrera, F.; Grimaldi, G.; Pinna-Susnik, M.; Murenu, A.; Rosboch, G.L.; Lybéris, P.; Ibba, F.; Balsamo, L.; et al. Lung Biopsy With a Non-intubated VATS Approach in an Obese Population: Indications and Results. Front. Surg. 2022, 9, 829976.
- Katgi, N.; Çimen, P.; Çirak, A.K.; Şimşek, T.; Ceylan, K.C.; Samancilar, Ö.; Duman, E.; Erer, O.F.; Tuksavul, F.F. Complication and cost analysis of transbronchial lung cryobiopsy and awake video-assisted thoracic surgery in diagnosis of interstitial lung disease. Sarcoidosis Vasc. Diffuse Lung Dis. 2022, 39, e2022005.
- Grott, M.; Wimmer, C.D.; Kreuter, M.; Prasse, A.; Eichhorn, M.E.; Eichhorn, F.; Herth, F.J.F.; Seeliger, B.; Kriegsmann, K.; Schmidt, W.; et al. Surgical Lung Biopsy for Interstitial Lung Disease: A Two Center Propensity Score Matching Analysis. Respiration 2022, 101, 910–917.
- Souza, J.M.; Pereira, I.R.P.D.; Borgmann, A.V.; Chiaradia, R.E.; Boscardim, P.C.B. Uniportal surgical biopsy, without orotraqueal intubation, without thoracic drainage in intersticial pulmonary disease: Initial results. Rev. Col. Bras. Cir. 2021, 48, e20202914.
- Peng, G.; Liu, M.; Luo, Q.; Chen, H.; Yin, W.; Wang, W.; Huang, J.; Qiu, Y.; Guo, Z.; Liang, L.; et al. Spontaneous ventilation anesthesia combined with uniportal and tubeless thoracoscopic lung biopsy in selected patients with interstitial lung diseases. J. Thorac. Dis. 2017, 9, 4494–4501.
- Rossi, G.; Spagnolo, P.; Wuyts, W.A.; Ryerson, C.J.; Valli, M.; Valentini, I.; Grani, G.; Gennari, A.; Bizzarro, T.; Lazzari-Agli, L. Pathologic comparison of conventional video-assisted thoracic surgical (VATS) biopsy versus non-intubated/“awake” biopsy in fibrosing interstitial lung diseases. Respir. Med. 2022, 195, 106777.
- Zhang, L.; Xie, T.; Li, Y.; Zhang, B.; Fu, Y.; Ding, Y.; Wu, H. Diagnostic value and safety of medical thoracoscopy under local anesthesia for unexplained diffuse interstitial lung disease: A retrospective study. Chron. Respir. Dis. 2022, 19, 14799731221133389.
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213.
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68.
This entry is offline, you can click here to edit this entry!
