Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Due to huge diversity and dynamic competition, the human gut microbiome produces a diverse array of antimicrobial peptides (AMPs) that play an important role in human health. The gut microbiome has an important role in maintaining gut homeostasis by the AMPs and by interacting with other human organs via established connections such as the gut–lung, and gut–brain axis. Additionally, gut AMPs play a synergistic role with other gut microbiota and antimicrobials to maintain gut homeostasis by fighting against multi-antibiotic resistance (MAR) bacteria.
- gut microbiota
- gut peptides
- multi-antibiotic resistance
- co-evolution
1. Introduction
The rapid emergence of multi-antibiotic resistance (MAR) and bacterial infections are global health concerns that urgently need to be addressed. The unavailability of new antibiotics and failure of available therapeutic strategies due to resistance development results in severe health complications and a sharp rise in deaths throughout the world [1][2]. In light of these facts, there is an urgent need for new antimicrobials and the development of new antimicrobial therapeutic strategies with effective outcomes to win the battle against MAR. Antimicrobial peptides (AMPs) are one of the promising options to fight against MAR due to their ubiquitous availability and diverse activity spectrum [3][4]. Additionally, the amenability of AMPs to bioengineering and drug repurposing may also play an important role in the development of new strategies to treat MAR [5][6][7]. Interestingly, the human gut is a complex environment where the cohabitation of pathogens with a beneficial gut microbiome and host appeases the synergistic co-evolution and action of gut AMPs and antibiotics. AMPs are also known to have multiple antimicrobial properties within a single peptide including membrane permeabilization and inhibition of both transcription and translation [8]. In the complex environment of the gut, high antimicrobial strength and complexity are observed in the tightly synchronized secretion of AMPs enriched with interdependent properties [9]. Host defense peptide-producing cells in the gut also take advantage of this synergistic action of gut AMPs in specific combinations that result in higher efficiency against pathogens at low concentrations. Similarly, the synergistic action of gut AMPs is observed with conventional antibiotics and could be used to develop new therapeutics against MAR. Interestingly, because of their known benefits, AMP-based drugs are now under consideration by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) [10][11]. Although gut AMPs have already been discussed extensively as a potential alternative to fighting against MAR, new strategies are required to control the development and evolution of rapid resistance [12][13][14].
2. Influence of Antibiotics on Gut Microbiota, Susceptibility to Infections, and Resistance Development
The most frequent and significant factor altering the normal gut microbiome composition and function is the use of antibiotics; however, many other factors that might impair the beneficial gut microbiota include mental and physical stress, radiation treatment, altered gut peristalsis, gastrointestinal infections, and dietary changes [15]. Antibiotics have a major impact on changing the gut microbiota, resulting in decreased bacterial diversity and increased numbers of some taxa [16]. This change in gut microbiome further results in the altered production of AMPs produced by gut microbiota and their associated functions impacting host immunity. Additionally, antibiotics’ activity spectrum, mode of action, potency, pharmacokinetics, dosage, and length of administration are also major factors that influence the gut AMPs and microbiome [17]; however, the presence of preexisting antimicrobial resistance genes in an individual’s microbiome is another concerning factor. Changes in the variety of gut bacteria can result in Clostridium difficile infection, which is naturally resistant to many antibiotics [18].
Antibiotics affect the local gut immune system by changing the composition of the gut resident microbiota and their metabolites, specifically AMPs. It has been shown that post-antibiotic treatment, the small intestine showed lower IL-17 and INF-γ production, while the colon showed decreased numbers of Treg cells. This suggests that antibiotics induce altered host–microbiota interactions that cause immune imbalance [19].
3. Interplay of Gut Microbiota with Gut AMPs
Gut microbiota plays an essential role in the regulation of the host defense system by maintaining gut homeostasis. Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria form the majority of the gut microbiome [20]. The diverse array of gut AMPs produced by gut microbiota plays an important role in various functional activities in the gut such as immunomodulatory activities and protection against pathogens by disrupting bacterial cell membranes and halting the RNA and DNA synthesis of metabolism [21]. Bacteriocins are the major bacterially produced gut AMPs and efficiently compete with other microbes in the gut. However, much of the gut microbiome’s diversity is still unknown; a study of some isolated microbes and metagenomic analysis suggested that there are many unrevealed classes of antibiotics and AMP-producing microbes present in the gut that are as yet unknown [22]. Gut microbiota-derived AMPs have been reported to protect against various disease-causing pathogens in the human gut. A bacteriocin, Abp118, produced by a gut microbe Lactobacillus salivarius UCC118 in the gut is confirmed to protect against the foodborne pathogen Listeria monocytogenes. It has been confirmed that mutant L. salivarius UCC118, expressing the cognate Abp118 immunity protein AbpIM, failed to protect against L. monocytogenes infections in mice [23]. Another bacteriocin, thuricin CD, produced by Bacillus thuringiensis DPC 6431 has been shown to have efficient killing potential against disease-causing clinical isolates of C. difficile without any antagonistic effect on commensal gut microbiota [24]. Bacteriocin encoded by pheromone-responsive plasmids is common in enterococcus strains residing in the gut which are reported as gut commensals as well as for casing hospital-acquired infections [25]. Bacteriocin 21, produced by conjugative plasmid pPD1 of Enterococcus faecalis, is demonstrated to protect against vancomycin-resistant enterococci without affecting the other commensal microbiota in the gut. Interestingly, E. faecalis containing pPD1 plasmid outcompetes and replaced other E. faecalis lacking pPD1. This suggests that gut bacteriocin can also regulate the niche in the gut and can be used as potential therapeutic peptides able to target MAR bacteria specifically [26].
Gut-epithelium-derived peptides are also reported to have potential antimicrobial activities against gut pathogens. In the gastrointestinal tract, enterocytes and Paneth cells are the primary cells responsible for the production of AMPs; however, macrophages, dendritic cells, neutrophils, and lymphocytes present in the lamina propria can also produce AMPs [27][28]. Defensins are the major AMPs secreted within the intestinal mucosa. The α and β defensins are abundant AMPs in the gut which are primarily secreted by Paneth and epithelial cells, respectively, in the intestine and the colon [29].
4. Synergistic Action of Gut AMPs with Conventional Antibiotics
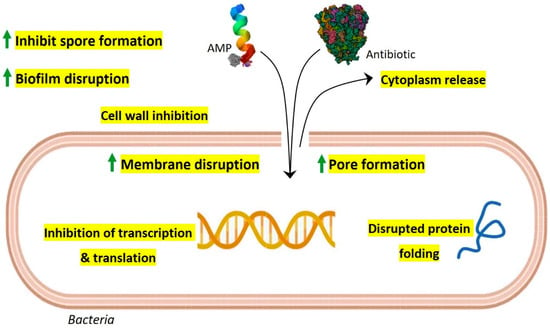
Due to the rapid emergence of multidrug resistance and the reduced efficacy of conventional antibiotics, the synergistic action of gut AMPs with antibiotics is explored and suggested as a new approach to control drug-resistant bacteria. Interestingly, AMPs display multiple mechanisms of action at a time that include membrane pore formation, inhibition of cell wall synthesis, biofilm disruption, inhibition of spore formation, and inhibition of protein synthesis and folding, along with inhibition of DNA and RNA synthesis [12]. Especially in the complex gut environment with the possibility of numerous unknown interactions, the multiple-mode-of-action scenario of AMPs is intriguing. The synergistic action of conventional antibiotics with gut AMPs is possibly benefited by extended pore opening on the target cell membrane, increased membrane permeabilization, and subsequently increased repair time that further results in altered bacterial intracellular functions and overall bactericidal activity (Figure 1).

Figure 1. Possible model for synergistic antimicrobial activity of gut AMPs with conventional antibiotics. As per their membrane-acting properties, continuous pore formation and increased membrane permeabilization by AMPs allow more influx of antibiotics and AMPs which results in efficient bactericidal activity along with improved targeting of intracellular components such as transcription, protein synthesis machinery, and protein folding. Gut AMPs might also facilitate enhanced biofilm disruption and inhibition of spore formation when used in combination with antibiotics.
5. Gut AMPs, Conventional Antibiotics, and Evolution of Resistance Development
A major global public health concern is bacterial resistance to small-molecule antibiotics that are already on the market. The global spread of antibiotic-resistant bacteria has created the possibility of a post-antibiotic age in which ordinary illnesses and small wounds could develop into potentially fatal conditions. Such resistance has resulted in the creation of multidrug-resistant bacteria over the past few decades, which can both endanger healthy people and cause serious infections in immunocompromised patients.
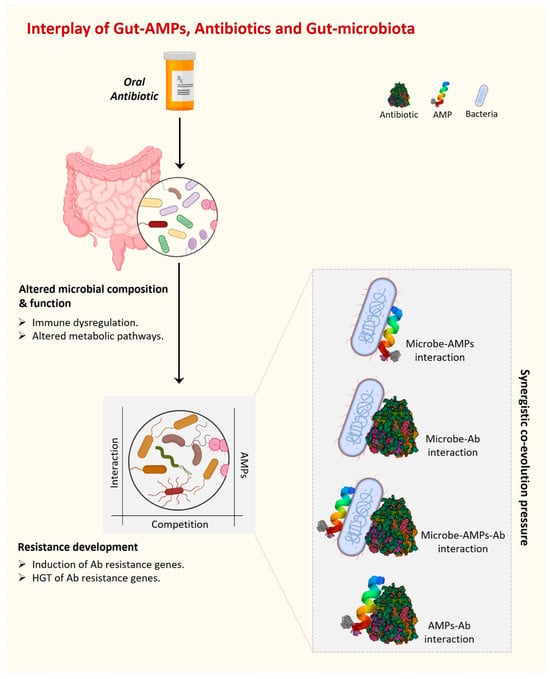
Antibiotic misuse has contributed to the emergence of MAR organisms. MAR infections are a leading source of morbidity and mortality worldwide [30]. Microbes can create and use defense and resistance mechanisms against the substances used to eradicate them in a complex environment such as the human gut, which is the home of over 100 trillion bacteria. Interestingly, not only the external antibiotics but also the antimicrobial substances produced by competitors present a challenge for the gut resident bacterial community to survive. Further, in the case of dysbiosis, an additional competition force exists between beneficial and harmful gut microbiota. AMPs are one such strategy used by bacteria (beneficial or harmful) to kill their competitors present in the surrounding complex environment. In addition to all of this, host-gut-derived AMPs are also present in the gut under the regulatory pressure of foreign AMPs, antibiotics, and the presence of their producers. Overall, there are multiple dynamic interactions present in the complex environment of the gut between various gut AMPs and antibiotics, whether internal or external. Together, these dynamic interactions and regulatory pressures create an evolutionary force under which microorganisms acquire a fair chance to evolve survival strategies and eventually develop antibiotic resistance (Figure 2).

Figure 2. The interplay of gut AMPs, antibiotics, and gut microbiota is driven by various interactions among them. These interactions develop a synergistic co-evolutionary pressure under which gut AMPs are co-evolved to fight against MAR.
Natural gut resident bacteria, bacteria with acquired resistance genes, and acquired bacteria with resistance genes that do not typically colonize the gut are all included in the gut resistance reservoir [31]. Although it is uncommon, it is conceivable for resistance genes or virulence features to be transferred between pathogenic and non-pathogenic gut resident bacteria. The interesting question of how the resident gut bacteria and gut AMPs have maintained their efficiency through evolutionary timeframes is prompted by the growing issue of antibiotic-resistant bacteria. This question may have a partial explanation in the fact that there is a huge diversity of AMPs in the gut produced by intestinal epithelial cells as well as by healthy gut microbiota, decreasing the chance of combination resistance.
6. Antimicrobial Stewardship and Modulation of Gut AMPs as a Tool to Fight against Resistance Development
The rationalized use of antibiotics is an important aspect of fighting against antimicrobial resistance by maintaining gut homeostasis and reducing alterations to gut AMPs. The rationalized use of antibiotics includes the choice, dose, and duration of antibiotic therapy. It has been reported in several large meta-analysis studies that using antimicrobial stewardship programs resulted in a reduced number of infections with MAR organisms [32][33]. The type and spectrum of antibiotics employed are critical factors in the development of resistance in the targeted microorganisms. The majority of the commensal population is anaerobic; thus, inappropriate and extended usage of anti-anaerobic antibiotics has been linked to an increased risk of MAR [34][35]. It has been reported that the use of narrow-spectrum antibiotics in place of anti-anaerobic antibiotics is favorable to the human gut microbiota since fewer commensals are impacted [36][37].
Protein engineering techniques can be used to improve the bioavailability or efficacy of the AMPs because of their proteinaceous nature. It is possible to generate AMP versions that are resistant to enzymatic digestion. Also, using engineering peptidomimetics, new variants of AMPs could be generated with an altered number of charged amino acid residues with decreased hydrophobicity and cytotoxicity as well [38].
7. Conclusions
Combinatory use of AMPs produced by both host and gut microbiota with conventional antibiotics could result in synergistic actions in different ways. It has been predicted that every species contains a unique set of AMPs that are evolved to defend the host against the microorganisms they might encounter [39]. This phenomenon becomes more complex and functionally specific in the case of the gut. The human gut is inhabited by millions of commensals which constitute the specific set of bacteria for every individual that is further affected by dietary habits, environment, and many more factors. Interestingly, there is a highly competitive environment in the gut so gut microbes are known to produce AMPs with various biological activities including immunomodulatory activities. Along with AMPs produced by gut microbiota, there are multiple host AMPs secreted in the gut in the proximity of gut epithelium and gut microbiota. It is hypothesized that all the gut AMPs synergistically affect each other’s functions to drive complex gut functions such as regulation of gut homeostasis; however, the mechanisms of this are not fully understood. In addition to fighting against infectious pathogens, gut AMPs play an essential role in the regulation of bacterial symbionts and communities in the gut, thus maintaining a balance between health and pathogenic microbes [40].
The human gut and AMP-producing intestinal epithelium constantly face a challenging dynamic microbial environment and also produce various antimicrobials for their survival that eventually affect the overall gut immune response including the efficacy of antibiotics during infection. To meet this challenge of the dynamic microbiome of the gut, epithelial cells also produce a wide variety of AMPs that quickly kill or inactivate bacteria, while a similar action is performed by the gut commensals to maintain the healthy gut environment which is called homeostasis. However, how the gut immune system differentiates between the healthy and pathogenic microbiota is still not well understood and remains a question of further research. On the other hand, in addition to this internal healthy equilibrium within the gut immune system, antibiotic treatment during infection creates another challenge for gut homeostasis. While both gut epithelium and commensals bear the adverse effects of antibiotics, gut AMPs have enough of a chance to interact with antibiotics, which affects the treatment efficacy as well (Table 2). However, it is not clear how AMPs interact with antibiotics and what is the response of AMP-producing gut epithelium and commensals in this dynamic complex gut environment. The emerging picture is that epithelial AMPs influence the structure and location of gut commensals in addition to protecting against pathogen colonization and invasion in synergism with the AMPs produced by commensals. Overall, gut AMPs are evolved for their antimicrobial action, efficacy, and spectrum under synergistic co-evolution with host immunity and commensals, along with interactions with other AMPs and conventional antibiotics (Figure 2).
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12121732
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655.
- Ventola, C.L. The antibiotic resistance crisis: Causes and threats. Pharm. Ther. 2015, 40, 277–283.
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 40, 488–505.
- Baindara, P.; Chaudhry, V.; Mittal, G.; Liao, L.M.; Matos, C.O.; Khatri, N.; Franco, O.L.; Patil, P.B.; Korpole, S. Characterization of the antimicrobial peptide penisin, a class Ia novel lantibiotic from Paenibacillus sp. strain A3. Antimicrob. Agents Chemother. 2016, 60, 580–591.
- Josef, J. Drug repurposing to overcome microbial resistance. Drug Discov. Today 2022, 27, 2028–2041.
- Deslouches, B.; Montelaro, R.C.; Urish, K.L.; Di, Y.P. Engineered cationic antimicrobial peptides (eCAPs) to combat multidrug-resistant bacteria. Pharmaceutics 2020, 12, 501.
- Baindara, P.; Mandal, S.M. Antimicrobial Peptides and Vaccine Development to Control Multi-drug Resistant Bacteria. Protein Pept. Lett. 2019, 26, 324–331.
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ Biofilms Microbiomes 2018, 4, 9.
- Wang, S.; Thacker, P.; Watford, M.; Qiao, S. Functions of Antimicrobial Peptides in Gut Homeostasis. Curr. Protein Pept. Sci. 2015, 16, 582–591.
- Lewies, A.; Du Plessis, L.H.; Wentzel, J.F. Antimicrobial Peptides: The Achilles’ Heel of Antibiotic Resistance? Probiotics Antimicrob. Proteins 2019, 11, 370–381.
- Costa, F.; Teixeira, C.; Gomes, P.; Martins, M.C.L. Clinical application of AMPs. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1117, pp. 281–298.
- Baindara, P.; Ghosh, A.K.; Mandal, S.M. Coevolution of Resistance Against Antimicrobial Peptides. Microb. Drug Resist. 2020, 26, 880–899.
- El Shazely, B.; Yu, G.; Johnston, P.R.; Rolff, J. Resistance Evolution Against Antimicrobial Peptides in Staphylococcus aureus Alters Pharmacodynamics Beyond the MIC. Front. Microbiol. 2020, 11, 103.
- Dinata, R.; Baindara, P. Laterosporulin25: A probiotically produced, novel defensin-like bacteriocin and its immunogenic properties. Int. Immunopharmacol. 2023, 121, 110500.
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543.
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39.
- Rafii, F.; Sutherland, J.B.; Cerniglia, C.E. Effects of treatment with antimicrobial agents on the human colonic microflora. Ther. Clin. Risk Manag. 2008, 4, 1343–1357.
- Rashid, M.U.; Weintraub, A.; Nord, C.E. Effect of new antimicrobial agents on the ecological balance of human microflora. Anaerobe 2012, 18, 249–253.
- Ivanov, I.I.; de Frutos, R.L.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific Microbiota Direct the Differentiation of IL-17-Producing T-Helper Cells in the Mucosa of the Small Intestine. Cell Host Microbe 2008, 4, 337–349.
- Lee, J.-Y.; Tsolis, R.M.; Bäumler, A.J. The microbiome and gut homeostasis. Science 2022, 377, eabp9960.
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins-a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105.
- Donia, M.S.; Cimermancic, P.; Schulze, C.J.; Wieland Brown, L.C.; Martin, J.; Mitreva, M.; Clardy, J.; Linington, R.G.; Fischbach, M.A. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell 2014, 158, 1402–1414.
- Corr, S.C.; Li, Y.; Riedel, C.U.; O’Toole, P.W.; Hill, C.; Gahan, C.G.M. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 2007, 104, 7617–7621.
- Rea, M.C.; Sit, C.S.; Clayton, E.; O’Connor, P.M.; Whittal, R.M.; Zheng, J.; Vederas, J.C.; Ross, R.P.; Hill, C. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl. Acad. Sci. USA 2010, 107, 9352–9357.
- Gilmore, M.S.; Lebreton, F.; van Schaik, W. Genomic transition of enterococci from gut commensals to leading causes of multidrug-resistant hospital infection in the antibiotic era. Curr. Opin. Microbiol. 2013, 16, 10–16.
- Kommineni, S.; Bretl, D.J.; Lam, V.; Chakraborty, R.; Hayward, M.; Simpson, P.; Cao, Y.; Bousounis, P.; Kristich, C.J.; Salzman, N.H. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature 2015, 526, 719–722.
- Filipp, D.; Brabec, T.; Vobořil, M.; Dobeš, J. Enteric α-defensins on the verge of intestinal immune tolerance and inflammation. Semin. Cell Dev. Biol. 2019, 88, 138–146.
- Phadke, S.M.; Deslouches, B.; Hileman, S.E.; Montelaro, R.C.; Wiesenfeld, H.C.; Mietzner, T.A. Antimicrobial peptides in mucosal secretions: The importance of local secretions in mitigating infection. J. Nutr. 2005, 135, 1289–1293.
- Dutta, P.; Das, S. Mammalian Antimicrobial Peptides: Promising Therapeutic Targets Against Infection and Chronic Inflammation. Curr. Top. Med. Chem. 2015, 16, 99–129.
- Chromek, M.; Arvidsson, I.; Karpman, D. The Antimicrobial Peptide Cathelicidin Protects Mice from Escherichia coli O157:H7-Mediated Disease. PLoS ONE 2012, 7, e46476.
- Anthony, W.E.; Burnham, C.A.D.; Dantas, G.; Kwon, J.H. The gut microbiome as a reservoir for antimicrobial resistance. J. Infect. Dis. 2021, 223, S209–S213.
- Baur, D.; Gladstone, B.P.; Burkert, F.; Carrara, E.; Foschi, F.; Döbele, S.; Tacconelli, E. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 990–1001.
- Ya, K.Z.; Win, P.T.N.; Bielicki, J.; Lambiris, M.; Fink, G. Association Between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, E2253806.
- Chanderraj, R.; Baker, J.M.; Kay, S.G.; Brown, C.A.; Hinkle, K.J.; Fergle, D.J.; McDonald, R.A.; Falkowski, N.R.; Metcalf, J.D.; Kaye, K.S.; et al. In critically ill patients, anti-anaerobic antibiotics increase risk of adverse clinical outcomes. Eur. Respir. J. 2023, 61, 2200910.
- Bhalla, A.; Pultz, N.J.; Ray, A.J.; Hoyen, C.K.; Eckstein, E.C.; Donskey, C.J. Antianaerobic Antibiotic Therapy Promotes Overgrowth of Antibiotic-Resistant, Gram-Negative Bacilli and Vancomycin-Resistant Enterococci in the Stool of Colonized Patients. Infect. Control Hosp. Epidemiol. 2003, 24, 644–649.
- Mitchell, B.G.; Hall, L.; White, N.; Barnett, A.G.; Halton, K.; Paterson, D.L.; Riley, T.V.; Gardner, A.; Page, K.; Farrington, A.; et al. An environmental cleaning bundle and health-care-associated infections in hospitals (REACH): A multicentre, randomised trial. Lancet Infect. Dis. 2019, 19, 410–418.
- Shahi, F.; Redeker, K.; Chong, J. Rethinking antimicrobial stewardship paradigms in the context of the gut microbiome. JAC—Antimicrob. Resist. 2019, 1, dlz015.
- Mojsoska, B.; Jenssen, H. Peptides and peptidomimetics for antimicrobial drug design. Pharmaceuticals 2015, 8, 366–415.
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395.
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368.
This entry is offline, you can click here to edit this entry!
