Cardiorenal syndrome type 4 (CRS type 4) occurs when chronic kidney disease (CKD) leads to cardiovascular damage, resulting in high morbidity and mortality rates. Mitochondria, vital organelles responsible for essential cellular functions, can become dysfunctional in CKD. This dysfunction can trigger inflammatory responses in distant organs by releasing Damage-associated molecular patterns (DAMPs). These DAMPs are recognized by immune receptors within cells, including Toll-like receptors (TLR) like TLR2, TLR4, and TLR9, the nucleotide-binding domain, leucine-rich-containing family pyrin domain-containing-3 (NLRP3) inflammasome, and the cyclic guanosine monophosphate (cGMP)–adenosine monophosphate (AMP) synthase (cGAS)–stimulator of interferon genes (cGAS-STING) pathway. Activation of these immune receptors leads to the increased expression of cytokines and chemokines. Excessive chemokine stimulation results in the recruitment of inflammatory cells into tissues, causing chronic damage. Experimental studies have demonstrated that chemokines are upregulated in the heart during CKD, contributing to CRS type 4.
1. Cardiorenal Syndrome Overview
The intrinsic association between cardiovascular disease (CVD) and kidney disease was first described by Robert Bright over a century ago [
1]. This maladaptive link is termed cardiorenal syndrome (CRS) [
2]. CRS comprises five distinct subtypes classified based on the initial pathology and temporality. Each subtype of CRS prevails due to the high global prevalence of both cardiac and renal diseases [
3,
4,
5,
6,
7]. Chronic kidney disease (CKD), for instance, affects approximately 10–12% of the world’s population [
6,
8] and ranks as the 12th leading cause of death. This predicament is projected to escalate due to aging, diabetes, and hypertension [
7]. CKD is characterized by persistent structural or functional renal alterations, often defined by a glomerular filtration rate (GFR) of less than 60 mL/min/1.73 m
2 [
9,
10].
CKD substantially augments the risk of cardiovascular mortality, with CKD patients facing a 10–30 fold higher risk of cardiac-related death, a risk that escalates with declining GFR [
19,
20,
21]. The primary cardiovascular complications and causes of mortality associated with CKD encompass ischemic heart disease, peripheral vascular disease, stroke, and heart failure [
7,
22,
23,
24]. In the context of CRS classification, CRS type 4 emerges when CKD plays a contributory role in CVD [
11,
25]. CRS type 4 poses a significant public health concern, yet its underlying pathophysiology remains inadequately understood [
17].
The Kidney-Heart Crosstalk
The causes of CRS type 4 are multifactorial and encompass a range of factors, including hemodynamic alterations, dysregulation of neurohormonal responses, overactivation of the renin–angiotensin–aldosterone system (RAAS), anemia, and pressure overload, among others [
11,
17,
27]. Additionally, as renal function declines, the accumulation of uremic toxins becomes a significant concern [
28,
29]. These factors have garnered attention for their roles in inducing cardiovascular alterations secondary to CKD.
Consequently, uremic patients often experience endothelial dysfunction, a prevalent complication of CKD [
30,
31]. Moreover, kidney–heart crosstalk is mediated by the systemic trafficking of extracellular vesicles (EV). This concept gains support from discovering EV-containing proteins in cardiac tissue that are not typically found in the heart but show increased presence in the kidney [
32]. Such interorgan communication can shed light on heart dysregulation in the context of CKD.
In response to proinflammatory insults, proximal tubule cells release exosomes, a specific type of EV, which carry dysregulated micro ribonucleic acids (RNAs) associated with the regulation of proinflammatory and profibrotic pathways, as well as dysregulated mitochondrial RNAs [
33]. As CKD progresses, uremic toxins continue to accumulate, exacerbating inflammation and oxidative stress in the kidney [
28,
34]. This accumulation likely induces inflammasome activation and pyroptosis by releasing cellular and mitochondrial components [
35]. Notably, mitochondrial components are identified as mitochondrial damage-associated molecular patterns (mtDAMPs), and they trigger inflammatory and immune responses, contributing to inflammation in various organs [
36,
37,
38].
Mitochondrial DAMPs stimulate innate immune signaling responses in different cardiac cell lineages, triggering the activation of transcription factors such as the nuclear factor-kappa B (NF-κB), a crucial factor for inflammation [
39,
40]. In addition, mitochondria provide an assembly platform for signaling innate immune responses, contributing to additional inflammatory responses [
41]. The main innate immune responses that are activated during the cardiorenal association include Toll-like receptors (TLRs), the nucleotide-binding domain-like receptor family pyrin domain-containing protein 3 (NLRP3) inflammasome, and cyclic guanosine monophosphate (cGMP)–adenosine monophosphate (AMP) synthase (cGAS)–stimulator of interferon genes (STING) [
42,
43,
44,
45,
46]. TLRs, NLRP3, and cGAS-STING pathways produce the upregulation of cytokines, vasoactive substances, chemokines, and inflammatory responses [
46,
47,
48]. Chemokine’s overstimulation produces the recruitment of leukocytes to tissues and dysregulated infiltration, leading to chronic cardiac damage [
49,
50,
51]. Interestingly, experimental studies have shown that chemokines are upregulated in the heart during CKD, establishing a link for CRS type 4 development [
52,
53].
Chemokine inhibitors have shown promise in reducing chronic inflammation and preventing cardiac and cardiorenal impairment [
52,
53,
54,
55]. Despite these advancements, the molecular mechanisms underlying how mtDAMPs are released by the kidneys in CKD may trigger innate immune pathways in the heart, ultimately leading to chemokine overactivation and the development of CRS type 4, remain poorly understood.
2. Mitochondrial Dysfunction and Inflammatory Alterations in CRS Type 4
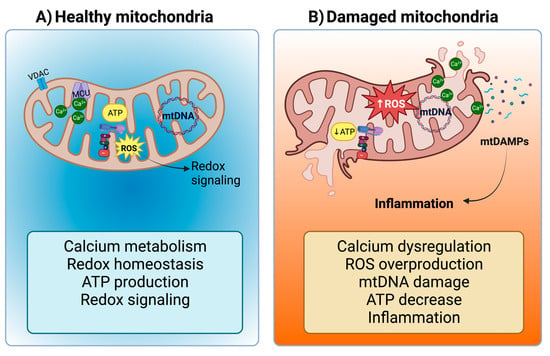
Mitochondria are versatile organelles with diverse roles encompassing biosynthesis, metabolism, calcium (Ca
2+) regulation, inflammation, and cell death, among other crucial cellular processes [
56]. Importantly, mitochondria are also the primary source of reactive oxygen species (ROS), particularly in complexes I and III of the electron transport system (ETS) [
57]. At moderate levels, these ROS function as secondary messengers, governing intracellular signal transduction cascades (
Figure 1A) [
58,
59,
60]. However, excess ROS production, often associated with reduced oxidative phosphorylation and ETS activity, leads to oxidative stress (
Figure 1B). Notably, due to their high energy demands, the heart and kidneys have a dense population of mitochondria [
58,
61,
62,
63]. Consequently, mitochondrial dysfunction serves as a potent trigger for the development of renal, cardiac, and cardiorenal diseases.
Figure 1. Mitochondria in tubular cells and cardiomyocytes. (A) Under normal conditions, mitochondria regulate calcium (Ca2+) metabolism (Ca2+ ions pass the mitochondrial outer membrane (MOM) through the Voltage-dependent Anion Channel (VDAC) and the mitochondrial inner membrane (MIM) through the mitochondrial calcium uniporter (MCU)), redox homeostasis, adenosine triphosphate (ATP) production, and reactive oxygen species (ROS) that act as second messengers for redox signaling. However, (B) mitochondrial damage leads to Ca2+ dysregulation, ROS overproduction, mitochondrial deoxyribonucleic acid (mtDNA) damage, ATP decrease, and finally, inflammation, the latter being induced by the release of mitochondrial damage-associated molecular patterns (mtDAMPs). Figure created by using Biorender.com.
Another critical characteristic of mitochondria is their ability to promptly detect and respond to insults through morphological changes, bioenergetic adaptations, self-renewal, and degradation [
63,
64]. However, in the context of CKD, fluid and electrolyte imbalance, retention of fluids, increased blood pressure, ROS overproduction, and hypertrophy instigate a cascade of mitochondria alterations, beginning in proximal tubular epithelial cells and subsequently affecting cardiomyocytes [
63,
65].
In the initial stages of inflammation, immune cells like neutrophils are recruited to phagocytose and clear dead cells and matrix debris, facilitating inflammation resolution despite the concomitant generation of ROS and inflammatory mediators [
69,
70,
71].
2.1. Mitochondrial Dysfunction in CKD Activates the NLRP3-NF-κB Pathway
In severe stages of CKD or during hemodialysis, elevated levels of the NLRP3 inflammasome and its activators, including uremic toxins, oxidative stress, and mitochondrial deoxyribonucleic acid (mtDNA), are observed in the serum or urine. These findings underscore the persistence of inflammation and fibrosis [
74,
75,
76]. Inflammasomes are cytoplasmic multi-protein signaling complexes that mediate the host’s immune response to cellular damage and infection [
77]. When NLRP3 is exposed to pattern-associated molecular patterns (PAMPs) such as viruses or bacteria, or DAMPs, it undergoes release and oligomerization through its central nucleotide-binding (NACHT) domain [
78]. The NLRP3 inflammasome triggers the activation of the transcription factor NF-κB [
79,
80,
81], thereby initiating additional inflammatory responses.
The NF-κB family encompasses distinct but related transcription factors, including p50, p52, p65 (RelA), c-Rel, and RelB [
81,
82]. These components form dimers and bind to specific DNA target sequences known as “κB” sites to modulate gene expression [
79,
81]. Among the many target genes under NF-κB’s control are cytokines (tumor necrosis factor-alpha (TNF-α), interleukins (IL-) IL-1β, IL-6, and IL-12), adhesion molecules, and some chemokines (CCL2, IL-18, CCL5, CXCL2, CXCL1, and CXCL10) [
83]. Importantly, the orchestration of NF-κB activation involves a critical role played by the NLRP3 inflammasome and ROS, which promote the phosphorylation of the p65 subunit, thereby activating NF-κB [
84].
Regardless of the underlying cause of CKD, renal mitochondria face challenges in meeting the increased demand for ATP [
85,
86]. This imbalance favors inorganic phosphate accumulation and increases oxygen uptake [
87,
88]. Notably, the reduction in adenosine triphosphate (ATP) production is consistently associated with lower oxidative phosphorylation (OXPHOS) due to decreased levels and/or activity of mitochondrial ETS complexes [
89,
90]. This decline in ETS activity, particularly in complexes I and III, augments mitochondrial ROS production and oxidative stress within these organelles [
91,
92].
In contrast, the renal succinate and fumarate levels increase in the unilateral ureteral renal obstruction (UUO) and chronic hypoxia CKD models, increasing ROS production [
97,
98]. Interestingly, the succinate accumulation induced by the hypoxia-inducible factor-1 alpha (HIF-1α) triggers macrophage stimulation, mediated by NLRP3 pathway activation, resulting in the rise of IL-1β and IL-18 secretion to the medium [
99].
In CKD, acetyl-CoA increase is associated with lipid intermediates and lipid derivatives accumulation in the kidney, leading to lipotoxicity [
102,
103]. Since early CKD stages, nephron segments like proximal tubules increase their lipid levels [
96,
102] and the fatty acid uptake protein CD36 levels [
102,
104], associated with inflammatory pathways induction [
105]. CKD lipotoxicity increases fatty acid levels in plasma and kidneys, especially palmitic acid [
103,
106,
107]. Palmitic acid has been shown to inhibit the adenosine monophosphate (AMP)-activated protein kinase (AMPK) signaling, reducing mitochondrial function, and increasing mitochondrial ROS production to favor the NLRP3 inflammasome activation, caspase-1, IL-1β, and IL-18 production [
107,
108]. This agrees with recent works suggesting that the early mitochondrial ETS alteration triggers the decrease in β-oxidation [
89,
93,
109]. Advanced stages of CKD are also characterized by the downregulation of β-oxidation enzymes in the kidney [
103,
110,
111], which induce inflammation in proximal tubules and glomeruli [
109,
112].
2.2. The Release of mtDAMPs during CKD and the Establishment of CRS Type 4
In the context of CKD, inflammation follows a distinct trajectory. Initially, it is characterized by functional alterations, including glomerular hyperfiltration, which serves as a compensatory mechanism [
113,
114]. However, structural changes emerge as time progresses, giving rise to a cascade of complications. These structural transformations encompass proteinuria, interstitial nephritis, tubular epithelial–mesenchymal transition, nephron fibrosis, and scarring [
65,
115,
116].
The structural changes are attributed to elevated circulating levels of various molecules, including fibrinogen, TNF-α, and IL-1β and IL-6. These molecules trigger an inflammatory response and promote the secretion of fibrotic mediators [
117,
118]. Consequently, inflammation leads to a reduction in mitochondrial ATP production, mitochondrial uncoupling, and an increase in ROS, culminating in oxidative stress and mitochondrial damage [
119,
120].
In response to mitochondrial uncoupling or oxidative stress, cells activate apoptosis, a programmed type of cell death. Apoptosis entails the release of mitochondrial proteins to the cytosol. This process is initiated by the oligomerization of effector Bcl2-family proteins such as B-cell lymphoma 2 (BCL-2)-associated X (BAX) and BCL-2 antagonist/killer 1 (BAK). These proteins form oligomers that promote the permeabilization of the mitochondrial outer membrane (MOM). As a result, mtDAMPs are released from both the intermembrane mitochondrial space and the matrix to the cytosol [
121,
122].
Another main form of cell death is necrosis, characterized by the plasma membrane’s rupture, which releases intracellular contents. The necrotic process is regulated, and it encompasses various types of necrosis, including ferroptosis, necroptosis, and pyroptosis. Each of these distinct mechanisms contributes significantly to various kidney diseases, either by directly affecting kidney cells or by recruiting immune cells and triggering inflammatory responses [
123].
2.3. NLRP3-NF-κB Pathway Activation in the Heart by CKD-Derived mtDAMPs and ROS
One of the different important stimuli for ROS production and immune activation in CKD occurs following the dissociation of the thioredoxin (TRX) complex from the thioredoxin-interacting protein (TXNIP), ultimately leading to the activation of the NLRP3 inflammasome [
135,
136]. TRX are ubiquitously present redox-active proteins known for their antioxidant and anti-inflammatory properties. Elevated ROS levels disrupt the TRX complex, causing TXNIP to bind to the leucine-rich repeat region of NLRP3, consequently activating the inflammasome [
137].
In the context of CVD, several CKD-related alterations, including increased levels of angiotensin II levels, ROS production, reduced activity of antioxidant enzymes, and inflammation, contribute to decreased levels of thioredoxins [
138,
139,
140]. Furthermore, the depletion of mitochondrial TRX2 in cardiomyocytes leads to hypertrophy and disrupts mitochondrial respiratory function by reducing AMPK activity [
141]. Impaired mitochondrial function and the activation of the NLRP3 inflammasome are associated with decreased levels of TRX2 during myocardial ischemia–reperfusion injury [
142]. Intriguingly, elevated levels of NLRP3 and IL-1β observed in patients with coronary artery disease exhibit an inverse association with the expression and protein levels of TXNIP and TRX1 [
143].
The rise in ROS levels due to disturbances in redox balance can also lead to the oxidation of mtDNA, a mtDAMP that activates the NLRP3 inflammasome [
146]. Additionally, ROS can directly activate NLRP3 inflammasome in CVD [
147]. These ROS may especially damage cardiomyocytes by activating the NF-κB pathway and the NLRP3 inflammasome [
148]. In addition, activation of the NLRP3-NF-κB-ROS pathway in CKD not only initiates inflammation but also triggers additional mechanisms contributing to cardiorenal disturbances. For instance, ROS levels may activate the transforming growth factor beta (TGFβ-1), a key player in cardiac fibrosis [
149]. TGFβ-1, in turn, further elevates ROS levels, promoting the activation of intracellular Smad pathways, leading to fibrosis, and decreasing antioxidant enzyme levels [
149,
150]. The consequence of reduced levels of antioxidant enzyme levels following kidney injury is the promotion of oxidative stress.
On the other hand, TNF-α, a pro-inflammatory cytokine known to induce cardiac hypertrophy, fibrosis, dysfunction under pressure overload, and chronic heart injuries [
151,
152], may trigger the activation of NLRP3 through the elevation of ROS levels [
153,
154]. This phenomenon can be elucidated by the chronic exposure of cells to TNF-α, which sets off p38-mitogen-activated protein kinases (MAPK) signaling, instigates inflammatory phenotypes, and suppresses the expression of antioxidant genes, resulting in an increase in ROS levels [
155].
Furthermore, the exposure of fibroblasts and human immune cells to TNF-α, in combination with oxidative stress, may prompt the degradation of the IκBα subunit by the IκB Kinase (IKK). This degradation event, in turn, leads to NF-κB activation and the transcription of genes associated with proinflammatory cytokines, chemokines, and NLRP3 inflammasome [
156,
157].
2.4. Involvement of NLRP3 Inflammasome and Toll-like Receptors 2 and 4 in CRS Type 4
Overactivation of NLRP3 inflammasome has been linked to myocardial fibrosis, hypertrophy, and cardiac dysfunction [
154,
161]. The upregulation of NLRP3, IL-1β, and IL-18 in the heart during CKD is closely associated with exposure to DAMPs, thus highlighting the cardiorenal connection [
146,
162].
DAMPs and mtDAMPs can trigger NLRP3 activation through TLRs, including uremic toxins, mitochondrial components released due to defects in membrane integrity, mitochondrial ROS, and cardiolipin [
45,
131].
TLRs are type I integral transmembrane proteins composed of three main components: an ectodomain with leucine-reach repeats (LRRs), a transmembrane domain, and a cytoplasmic Toll/IL-1 receptor (TIR) domain. LRRs recognize PAMPs or DAMPs, while the TIR domain initiates the downstream signaling [
128,
163]. The primary functions of TLRs include stimulating phagocytosis and mediating inflammation by sensing molecules from damaged cells [
164].
The activation of TLRs by ligands results in the dimerization of TLRs’ cytoplasmic signaling domains [
165]. This TIR-TIR complex initiates downstream signaling by recruiting specific adaptor molecules [
166]. DAMPs and mtDAMPs, such as debris from apoptotic and necrotic cells, inflammatory factors, nucleic acid fragments, oxidative products, and uremic toxins generated during renal damage can activate classical TLR2 and TLR4 pathways in the heart [
51,
71].
Upon recognition of DAMPs and mtDAMPs by TLR2 and TLR4, they stimulate macrophages to produce inflammatory cytokines [
145,
167,
168]. TLR2 and TLR4 rely on adaptor molecules, with TLR2 engaging myeloid differentiation factor 88 (MyD88) and TLR4 utilizing Toll/IL-1 receptor (TIR) domain-containing adaptor inducing interferon beta (TRIF) [
166]. Activation of the MyD88-dependent pathway involves the participation of IL-1 receptor-associated kinases (IRAK), including IRAK1 and IRAK4, TNF receptor-associated factor 6 (TRAF-6), and MAPK. These events culminate in the activation of the transcription factor NF-κB, leading to the production of proinflammatory cytokines such as pro-IL-1β and pro-IL-18 [
71,
169].
Sustained inflammation in the kidney may lead to the activation of TLR2 and TLR4 in the heart, thereby contributing to CRS type 4. Supporting this notion, the deletion of both TLRs during unilateral kidney ischemia/reperfusion has demonstrated a reduction in cardiac hypertrophy markers such as B-type natriuretic peptide and α-actin. This suggests that sustained inflammation in the kidney can upregulate TLRs in the heart [
170].
Another set of activators for TLR2 and TLR4 includes HSP proteins. HSPs are intracellular chaperones with a pivotal role in stress responses. In particular, HSP70 has been identified in the extracellular medium, where it is recognized as a DAMP and activates immune cells. This activation results in the secretion of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α [
172]. The release mechanism of extracellular HSP70 involves a membrane-associated form [
173]. In the context of CKD, HSP70 levels appear elevated in the urine and serum of patients, which is closely associated with inflammation and immune responses [
174,
175].
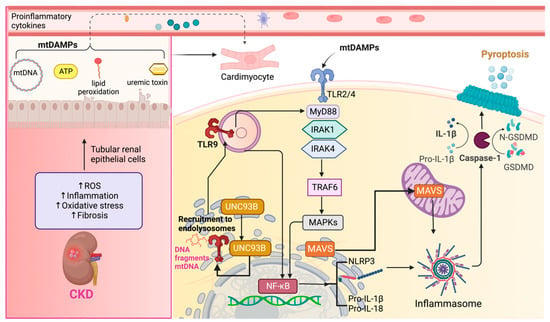
Therefore, maintaining regular levels of HSP90 is necessary to prevent cardiovascular disturbances. However, it is important to note that an excessive increase in this protein could also potentially favor the development of CRS type 4. In summary, TLR2 and TLR4 activation and downstream inflammatory signaling are central factors contributing to cardiac disorders during CKD, ultimately promoting the establishment of CRS type 4 (Figure 3).
Figure 3. Toll-like receptors (TLRs) in cardiorenal syndrome type 4 (CRS type 4). In chronic kidney disease (CKD), several factors contribute to the initiation of inflammation, oxidative stress, and fibrosis in renal tubular epithelial cells. These cellular insults result in the release of Damage-associated Molecular Patterns (DAMPs), including mitochondrial deoxyribonucleic acid (mtDNA), ATP, peroxidized lipids, and uremic toxins, which play a pivotal role in mediating inflammatory responses in cardiomyocytes and the recruitment of inflammatory cells. mtDNA, extracellular ATP, peroxidized lipids, and uremic toxins can activate membrane-bound Toll-like receptors, specifically TLR2 and TLR4. The activation of these receptors is mediated by the adaptor molecule myeloid differentiation factor 88 (MyD88). Once TLR2 and TLR4 are engaged, they initiate the mitogen-activated protein kinases (MAPKs) pathway. Activated MAPKs ultimately lead to the activation of nuclear factor kappa B (NF-κB), a transcription factor involved in the regulation of inflammatory genes. NF-κB promotes the assembly of the NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome. Within this complex, pro-interleukin-1 beta (pro-IL-1β) and pro-interleukin-18 (pro-IL-18) are processed to form the mature and active IL-1β and IL-18. TLR9 might be activated by internal, external, or mtDNA, which induces the recruitment of TLR9 by UNC93B protein from the endoplasmic reticulum to endolysosomes. It leads to the activation of MAPKs mediated by MyD88 by following the same steps of TLR2 and TLR4. The activation of NLRP3 results in a cascade of events, ultimately leading to pyroptosis. Pyroptosis is mediated by gasdermin D (GSDMD), which forms pores in the cell membrane, causing cell lysis and the release of pro-inflammatory intracellular contents. The mitochondrial antiviral proteins (MAVS) also activate NLRP3, leading to pyroptosis induction. IL-1 receptor-associated kinases (IRAK), IRAK1 and IRAK4, TNF receptor-associated factor 6 (TRAF-6). Figure created by using Biorender.com.
2.5. Role of TLR9 in Inflammation and Its Implication in CRS Type 4
Another TLR known to induce inflammation through the adaptor molecule MyD88 is TLR9 [
166]. TLR9, primarily localized in endolysosomes, is associated with activating p38 MAPK signaling [
179]. The inflammatory response initiated by TLR9 is triggered by DNA fragments rich in unmethylated cysteine–phosphate–guanine motifs, with mtDNA being a notable example [
180]. These DNA fragments can be internalized in various tissues by dendritic cells and macrophages [
162] and subsequently delivered to endolysosomes, where they activate TLR9 [
166].
Upon activation, TLR9 interacts with the endoplasmic reticulum (ER) membrane protein UNC93B, facilitating its transportation to the endolysosomal compartment [
181,
182] (
Figure 3). TLR9 activation leads to two distinct pathways [
183]: one is associated with the transcriptional activation of proinflammatory cytokines, requiring the involvement of NF-κB, while the other relates to the activation of type I interferon genes [
184].
Activation of TLR9 increases in renal proximal tubular cells following ischemic injury, initiating a cascade of events that promote inflammation, apoptosis, and necrosis through NF-κB and caspase-dependent pathways [
186]. Because of apoptosis or necrosis, renal DAMPs are released. These DAMPs may be exposed to the cell surface and released into the extracellular space, acting as potent inflammation triggers [
125].
Renal DAMPs have the potential to activate TLR9 in cardiac cells, inducing oxidative stress and inflammatory responses, which can lead to the release of mtDNA or other mtDAMPs within cardiomyocytes. This process has been observed in mice cardiomyocytes, where the release of mtDNA after myocardial injury activated NF-κB through TLR9, ultimately contributing to cell death [
187].
2.6. Extracellular Vesicles (EV) and Their Role in Inflammation
Exogenous mtDNA and other signaling molecules may also be transported into other cells, including cardiac cells, through extracellular vesicles (EVs). These EVs, such as exosomes, microvesicles, and apoptotic bodies, carry various cargo, including nucleic acids, proteins, and metabolic intermediaries [
190,
191].
Apoptotic bodies, in particular, contain fragments of nucleic acids, lipids, proteins, and organelles [
192]. Notably, apoptotic cells release ATP, which can serve as a signaling molecule by binding to purinergic receptors on cell membranes, activating intracellular signaling pathways, and potentially inflammasome assembly.
2.7. The Role of Autophagy and Mitophagy in NLRP3 Signaling Pathway in CRS Type 4
Autophagy consists of vesicular sequestration of cellular components, inducing their degradation and further recycling [
196]. This process comprises initiation, elongation, fusion, and degradation and is regulated by the phosphoinositide-3 kinase (PI3K) and Unc-51-like kinase (ULK) complexes [
197]. The activation of ULK depends on the AMPK protein that phosphorylates and inhibits the mammalian target of Rapamycin complex 1 (mTORC1). PI3K is activated after the autophagic protein Beclin is disassembled from Bcl2, forming the PI3K complex to produce phosphatidyl inositol triphosphate (PI3P).
On the other hand, mitophagy is a specialized form of autophagy that removes damaged mitochondria and is crucial for immune system vigilance and mitochondrial quality control. Mitophagy occurs when the mitochondrial membrane potential (ΔΨ) is disrupted and involves PTEN-induced kinase (PINK) and E3 ubiquitin ligase (Parkin) proteins. Both in autophagy and mitophagy processes, sequestosome p62 proteins (p62) are necessary for degradation because these proteins recognize and ubiquitinate damaged organelle and protein aggregates. Moreover, the microtubule-associated protein 1A/1B-light chain 3 (LC3) is involved in the elongation of autophagosomes [
198].
Mitophagy’s role in NLRP3 inflammasome activation is shown by removing autophagy-related proteins, causing the accumulation of damaged mitochondria, and increasing mtDAMPs production [
199]. For example, it has been demonstrated that NF-KB restricts NLRP3 inflammasome activation through p62-dependent mitophagy; conversely, the absence of p62 promotes greater mitochondrial damage and increased inflammation [
200].
2.8. MAVS and NLRP3-NF-kB Signaling in CRS Type 4
Another role of mitochondria in NLRP3 activation is associated with mitochondrial antiviral proteins (MAVS). MAVS comprises an N-terminal CARD-like domain and a C-terminal transmembrane domain, essential for MAVS signaling. Notably, the transmembrane domain targets MAVS to the mitochondria in the MOM [
207]. The latter allows MAVS to participate in the relocalization and association of NLRP3 with ER and mitochondria organelle clusters [
137]. This facilitates NLRP3 oligomerization [
208,
209].
Low active caspase-1, IL-1β, and IL-18 levels induce cytokine production, but higher levels of these molecules can induce cell death by apoptosis or pyroptosis. When NLRP3 is activated and associated with MAVS, it leads to pyroptosis [
211].
Pyroptosis is a caspase-1-dependent death mediated by the cleavage of gasdermin D by caspase-1 and the subsequent formation of stable pores in the cell membrane [
212,
213]. The pores formed by gasdermin D proteins promote cell swelling and lytic cell death, releasing cytosolic contents into the extracellular space that act as DAMPs [
214]. Also, pyroptosis is regulated through the NLRP3 inflammasome [
215]. It could be associated with the release of DAMPs from renal cells, which may activate inflammatory processes in other organs, such as the heart.
3. The Role of the cGAS-STING Pathway in CRS Type 4
3.1. The cGAS-STING Pathway
The cGAS-STING pathway plays a pivotal role in mediating inflammation in response to infections, cellular stress, and tissue damage [
218]. cGAS activity is triggered by interactions with various ligands, including double-strand DNA (dsDNA), neutrophil DNA–protein complexes, and mtDNA in mammals [
218,
219]. When cGAS interacts with these ligands, it generates a product known as 2′3′cyclic GMP-AMP [
220]. This cyclic GMP-AMP molecule then binds to the STING protein located in the ER membrane [
221].
The downstream signaling cascade begins with the translocation of STING from the ER to the Golgi apparatus, facilitated by the ER-to-Golgi transport machinery, specifically the ER–Golgi intermediate compartment (ERGIC) [
218,
221]. This translocation of STING is a critical step in activating the immune signaling pathway [
222,
223]. Once in perinuclear compartments, STING forms a complex with TRAF family member-associated NF-κB activator (TANK)-binding kinase (TBK1) [
224]. TBK1, in turn, phosphorylates transcription factors, including the interferon regulatory factor 3 (IRF3) and NF-κB [
218].
3.2. The Activation of the cGAS-STING-NF-κB Axis by mtDNA Release in CKD
CKD has been strongly linked to the activation of the cGAS-STING pathway. For instance, in a study conducted by Chung et al. [
226], a positive correlation was observed between CKD-induced fibrosis and the expression of cGAS and STING in over 400 kidney tissue samples. Experimental models of diabetic kidney disease and Alport syndrome have shown that the cGAS-STING pathway plays a significant role in the development and progression of glomerular damage by regulating inflammation [
227]. Specifically, this pathway is associated with cell damage and chronic inflammation, resulting in the production of inflammatory cytokines and interferons [
228].
In CKD, an oxidative stress state is closely related to renal functional and structural alterations, primarily through mitochondrial dysfunction and increased production of ROS [
8]. Notably, the plasma of patients receiving platinum-based nephrotoxic anticancer therapy showed elevated levels of mtDNA in plasma, suggesting that STING signaling might be activated through this mechanism [
229].
The generation of ROS and Ca
2+ ion accumulation can trigger the opening of the mitochondrial permeability transition pore (mPTP), resulting in the loss of ΔΨ, uncoupling of the ETS, and the release of proapoptotic factors like cytochrome c, which can lead to apoptosis or necrosis. During apoptosis, macropores form in the MOM due to the regulation of BAX and BAK [
231,
232,
233]. These BAX-mediated pores in the MOM allow the inner membrane to herniate, leaking mtDNA and other mitochondrial matrix components in the cytoplasm.
In the context of cisplatin-induced nephrotoxicity, it has been suggested that mitochondrial permeabilization induced by BAX pores in the MOM can activate the cGAS-STING pathway, thus triggering inflammation [
225]. Small-molecule STING inhibitors, such as H151, have shown promise in ameliorating renal function, kidney morphology, inflammation, and mitochondrial alterations following cisplatin-induced nephrotoxicity [
229]. Additionally, activation of the cGAS-STING pathway has been observed in diabetic kidney disease resulting from mitochondrial damage [
235].
3.3. The Activation of the cGAS-STING-NF-κB Axis by mtDNA Release in CRS Type 4
Activation of the immune response in CRS type 4 has been linked to the escape of mtDNA into the cytoplasm, thereby triggering the cGAS-STING pathway [
226]. In experimental diabetic cardiomyopathy, the release of mtDNA into the cytosol of heart cells induces inflammation through the cGAS-STING pathway, activating downstream genes, including IRF3, NF-κB, IL-18, and IL-1β [
219]. IL-1β, in particular, can potentially disrupt mitochondrial homeostasis by amplifying immune reactions through its activation of cGAS via mtDNA [
244,
245].
In experimental models of uremic cardiomyopathy, mitochondrial oxidative stress emerges as a consequence of CKD. Oxidative stress triggers the voltage-dependent anion channel (VDAC)-mediated MOM permeabilization, leading to the release of mtDNA and subsequently activating the STING-NF-κB pathway within the heart [
41]. DNA fragments released from metabolic organs, originating from the body’s own cells, promote chronic inflammation as they serve as endogenous ligands for the cGAS-STING pathway [
227].
4. Chemokines Activation and the Pathophysiology of CRS Type 4
4.1. Chemokines Overview
In the context of the heart, inflammation resulting from a uremic state and mitochondrial dysfunction often leads to endothelial dysfunction, oxidative stress, atherosclerosis, vascular calcification, and progressive tissue damage [
248,
249]. This suggests that the dysregulation of NF-κB via TLRs, NLRP3, and cGAS-STING could serve as a mechanism underlying chronic heart inflammation and the overproduction of chemokines in CRS type 4.
Chemokines are small-molecular-weight chemotactic cytokines [
250] that play pivotal roles in directing the migration of neutrophils and monocytes during both acute and chronic inflammation [
251]. These chemokines are classified into the following subfamilies, including CXC, CC, XC, and CX3C, based on the position of conserved cysteine residues in their N-terminal domain [
252].
CC chemokines, characterized by two adjacent cysteine residues, primarily attract monocytes and macrophages through distinct receptors [
254]. On the other hand, CXC chemokines feature two cysteine residues separated by a single amino acid (C-X-C) [
255,
256]. The transcription of certain chemokines is modulated by NF-κB, depending on regulatory elements, including the adjacent activating protein 1 and C/EBP elements.
4.2. The Role of Chemokines and Receptors in the Pathophysiology of CKD
In a healthy kidney, various cell types, including endothelial cells, podocytes, mesangial cells, tubular epithelial cells, and interstitial fibroblasts, typically produce low levels of inflammatory chemokines [
260,
261]. In patients with CKD, these chemokines are predominately induced by pro-inflammatory cytokines and ROS [
262].
The primary role of chemokines in the kidney is to facilitate the recruitment of leukocytes and T cells, which play a central in interstitial fibrosis and the progression of CKD [
260,
263]. Other factors contributing to chemokine activation in CKD include uremic toxins, cyclic adenosine monophosphate (cAMP), growth factors, lipopolysaccharides, low-density lipoprotein (LDL), IFN-γ, and vasoactive substances [
253,
262,
264]. These factors can further upregulate chemokines by influencing NF-κB and other transcription factors [
150]. Consequently, an excess of damaging stimuli in CKD can lead to the overstimulation of chemokines, accelerating disease progression.
4.2.1. Monocyte Chemoattractant Protein-1 (MCP-1)/CCL2 and CCR2 Receptor in CKD
CCL-2 is a well-studied chemokine in cardiac and renal diseases, known for its ability to attract monocytes, T lymphocytes, and natural killer cells [
250]. Excessive activation of CCL2 leads to an overwhelming cellular infiltration and prolonged inflammatory response, exacerbating tissue damage and affecting kidney function [
265,
266]. Upregulation of CCL2 by NF-κB has been linked to tubulointerstitial injury in proteinuric renal disease [
267]. Conversely, reducing protein accumulation in renal disease has been shown to decrease CCL2 levels [
268].
In advanced CKD, the TGF-β/Smad2,3 pathway activation induces CCL2 expression in renal cells, resulting in a chemotactic effect on macrophages [
269]. Likewise, in the UUO model, a well-established model for studying fibrosis in CKD, a wide expression of CCL2 is observed, leading to macrophage infiltration, tubulointerstitial CCL2 expression, leading to macrophage infiltration via a TGF-β/Smad3-dependent signaling pathway [
270,
271]. Therefore, CCL2 plays a pivotal role in progressive interstitial fibrosis in CKD.
4.2.2. C-C Motif Chemokine 8 (CCL8/MCP-2) in CKD
CCL8 is a CC chemokine that plays a pivotal role in attracting inflammatory monocytes and T lymphocytes in various pathological conditions [
278,
279]. In advanced CKD and fibrosis-related human glomerulopathies [
280], CCL8 levels significantly increase, primarily due to the activation of the TGF-β pathway. Consequently, inhibiting CCL8 has been proposed as a preventive therapy against fibrosis in CKD. In the mice-UUO model, functional blockade of CCL8 with a monoclonal antibody has been shown to prevent fibrosis and apoptosis in renal cells [
50].
4.2.3. Chemokine Interferon-γ-Inducible Protein 10 (IP-10)/Chemokine (C-X-C Motif) Ligand (CXCL)10 in CKD
CXCL10, a member of the CXC chemokine family, exerts its biological functions by binding to the CXCR3 receptor [
283]. CXCR3 is expressed in T lymphocytes, natural killer (NK) cells, inflammatory dendritic cells, macrophages, and B cells [
284]. CXCL10 is involved in chemotaxis, apoptosis induction, cell growth regulation, and angiostatic effects. It is primarily secreted by leukocytes, activated neutrophils, eosinophils, epithelial cells, and endothelial cells in response to IFN-γ [
273]. Once activated, CXCL10 attracts Th1 lymphocytes, monocytes, T cells, and NK cells [
273,
283]. Interstitial CXCR3 has been implicated in the progressive loss of renal function in human glomerular diseases [
260].
As a consequence of CKD, inflammatory processes can also manifest in the heart, resulting in significant alterations, including heart failure, coronary artery disease, arrhythmias, and sudden cardiac death. This can ultimately lead to the development of CRS type 4.
5. Conclusions
CKD induces hemodynamic and metabolic changes that lead to mitochondrial damage, causing the release of various components into the peripheral circulation. These mitochondrial components activate inflammatory signaling pathways in organs like the heart, resulting in the upregulation of inflammatory genes, including chemokines and cytokines, further exacerbating damage. Chemokines play a pivotal role in attracting inflammatory cells, thereby intensifying inflammation, and contributing to the development of CRS type 4 development.
This entry is adapted from the peer-reviewed paper 10.3390/ijms242115875