Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Coatings & Films
Graphitized carbon nitride (g-C3N4), as a metal-free, visible-light-responsive photocatalyst, has a very broad application prospect in the fields of solar energy conversion and environmental remediation. The g-C3N4 photocatalyst owns a series of conspicuous characteristics, such as very suitable band structure, strong physicochemical stability, abundant reserves, low cost, etc. Research on the g-C3N4 or g-C3N4-based photocatalysts for real applications has become a competitive hot topic and a frontier area with thousands of publications over the past 17 years.
- photocatalyst
- g-C3N4
- reaction parameters
- structure design
- exfoliation
1. Introduction
Recently, graphitic carbon nitride (g-C3N4) has attracted extremely wide attentions in photocatalysis due to its special band structure, stable properties, low price, and easy preparation [2,3,4,5,6]. The g-C3N4 is comprised of only carbon and nitrogen elements, which are very abundant on the Earth. Importantly, the g-C3N4 materials can be easily fabricated by thermal polymerization of abundant nitrogen-rich precursors such as melamine [7,8,9,10,11,12,13,14,15,16], dicyandiamide [17,18,19,20,21,22], cyanamide [23,24,25], urea [18,26,27], thiourea [28,29,30], ammonium thiocyanate [31,32,33], etc. Because the band gap of g-C3N4 is 2.7 eV, it can absorb visible light shorter than 450 nm effectively, implying broad prospects in solar energy conversion applications. Due to the aromatic C-N heterocycles, g-C3N4 is thermally stable up to 600 °C in air. Moreover, g-C3N4 is insoluble in acids, bases or organic solvents, exhibiting good chemical stability.
However, some bottlenecks in the photocatalytic activity of g-C3N4 still exist, such as fast photogenerated carrier recombination, limited active site, small specific surface area, low light absorption capacity, unsatisfactory crystallinity and unignorable surface defects. How to promote the efficient migration and separation of photogenerated carriers, expand the spectral response range and increase the specific surface area of g-C3N4 is the core problem to achieve high energy conversion efficiency. In practice, the introduction of impurities into the g-C3N4 matrix through copolymerization and doping has become an effective strategy to change the electronic structure and band structure of g-C3N4. On the other hand, numerous research works have demonstrated that the physicochemical properties and photocatalytic efficiency of the polymer g-C3N4 can be significantly improved by optimizing synthesis techniques such as supramolecular and copolymerization techniques with identical structural and nano-structural designs, or by template-assisted methods to improve porosity and surface area [34,35,36,37,38,39]. Amongst various modification approaches, designing and constructing a more suitable band structure is the most important prerequisite to improve the charge separation efficiency, thereby enhancing the photocatalytic performance.
2. Influence of Synthesis Parameters
2.1. Precursors and Reaction Temperature
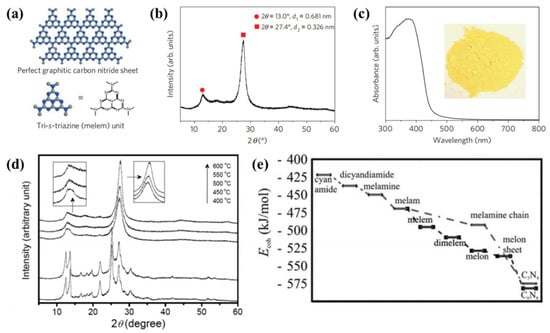
The first reported g-C3N4 as a heterogeneous catalysis was present in 2006 [40]. Subsequently, various precursors such as urea [41], thiourea [42], cyanamide [43,44] and dicyandiamide [45,46] have been employed to synthesize g-C3N4 by thermal treatment methods. In 2009, Wang et al. firstly used cyanamide as the precursor of g-C3N4 for producing hydrogen from water under visible-light irradiation in the presence of a sacrificial donor [47]. This pioneering work represents an important first step towards photosynthesis in general, where artificial conjugated polymer semiconductors can be used as energy transducers. In order to demonstrate the reaction intermediate compounds, characterization techniques such as thermogravimetric analysis (TGA) and X-ray diffraction (XRD) are used to characterize the reaction. Figure 2a displayed that the graphitic planes are constructed from tri-s-triazine units connected by planar amino groups. Figure 2b is the XRD pattern of the obtained g-C3N4 powder. From the ultraviolet-visible spectrum (Figure 2c), it can be seen that the band gap of g-C3N4 is 2.7 eV. The synthesis of g-C3N4 was a combination of polyaddition and polycondensation. At a reaction temperature of 203 and 234 °C, the cyanamide molecules can be condensed to dicyandiamide and melamine, respectively. The ammonia is then removed by condensation. When the temperature reaches 335 °C, large amounts of melamine products are detected. When further heating to 390 °C, the rearrangements of melamine will result in the formation of tri-s-triazine units. Finally, when heating to 520 °C, the polymeric g-C3N4 are synthesized via the further condensation of the unit. However, g-C3N4 will be unstable at above 600 °C. Figure 2d shows the structural phase transition process from cyanamide to g-C3N4 at different temperatures. In addition to in-situ characterization experiments to verify the reaction process, it can also be demonstrated by relevant simulation calculations. The first-principles DFT calculations were performed using a plane wave basis set with a 550 eV energy cutoff [40]. The calculation results showed that the cohesion energy increased under the addition of multiple reaction pathways, which confirmed that melamine was produced upon heating the cyanamide, as shown in Figure 2e.

Figure 2. Crystal structure and optical properties of g-C3N4. (a) Schematic diagram of a perfect g-C3N4 sheet constructed from melem units. (b) Experimental XRD pattern of polymeric carbon nitride, revealing a graphitic structure with an interplanar stacking distance of the aromatic unit (0.326 nm). (c) Diffuse reflectance spectrum of the polymeric carbon nitride. Inset: Photograph of the photocatalyst. (d) XRD patterns of g-C3N4 treated at different temperatures. (e) Calculated energy diagram for the development of g-C3N4 using cyanamide precursor [47].
2.2. C/N Ratio
Generally, g-C3N4 exhibits a high physicochemical stability and ideal band structure, due to the high condensation degree and the presence of the heptazine ring structure. When the appropriate precursor and condensation method are selected, the C/N ratio in layered g-C3N4 is about 0.75. Many studies have confirmed that when the precursors and synthesis parameters of g-C3N4 are changed, the physicochemical properties of g-C3N4 will be significantly affected, such as band gap width, specific surface area, C/N ratio, etc., which will directly affect the photocatalytic efficiency and other applications’ performance [50,51,52,53,54]. Yan et al. synthesized g-C3N4 by directly heating the low-cost melamine, and they change the C/N ratio by controlling different heating temperatures [55]. The research showed that when the heating temperatures increased from 500 to 580 °C, the ratio of C/N increased from 0.721 to 0.742. Meanwhile, the band gaps of g-C3N4 decreased from 2.8 to 2.75 eV.
2.3. Pretreatment of Precursors
It has been shown that modification and pretreatment of nitrogen-rich precursors before thermal annealing can effectively improve the physicochemical properties of g-C3N4. One of the effective pretreatment methods is by acid treatment. Yan et al. reported the synthesis of g-C3N4 by directly heating the sulfuric-acid-treated melamine precursor [59]. It is worth noting that the carbon nitride synthesized from sulfuric acid treated melamine (15.6 m2/g) shows relatively higher BET surface area than that of samples synthesized from untreated melamine (8.6 m2/g). The reason can be attributed to the effect of pretreatment of melamine with H2SO4 on its condensation process, during which sublimation of melamine is inhibited significantly. In addition to the pretreatment of melamine with H2SO4, HCl and HNO3 also exhibited good pretreatment effects on melamine [60,61,62,63].
In addition to acid precursors, pretreatment methods of sulfur-mediated synthesis can also be used to regulate the structure and physicochemical properties of g-C3N4 [64]. The fundamental reason is that the presence of the sulfur group in the sulfur-containing thiourea provides an additional chemical pathway to regulate the degree of condensation and polymerization of g-C3N4 because it is easy to leave the -SH groups.
2.4. Reaction Atmosphere
In addition to the types of precursors, reaction temperature and duration, the physicochemical properties and structure of g-C3N4 are also strongly influenced by the reaction atmosphere, because the reaction atmosphere can induce a variety of defects and carbon and nitrogen vacancies. In fact, defects are essential for catalytic reactions because they can act as active sites for reactant molecules and change the band structure by introducing additional energy levels in the forbidden band, thus extending the spectral absorption range [66,67,68,69]. By controlling the polycondensation temperature of a dicyandiamide precursor in the preparation of g-C3N4, Niu et al. introduced nitrogen vacancies in the framework of g-C3N4 [70]. The excess electrons caused by nitrogen loss in g-C3N4 lead to a large number of C3+ states associated with nitrogen vacancies in the band gap, thus reducing the intrinsic band gap from 2.74 eV to 2.66 eV. Steady and time-resolved fluorescence emission spectra show that, due to the existence of abundant nitrogen vacancies, the intrinsic radiative recombination of electrons and holes in g-C3N4 is greatly restrained, and the population of short-lived and long-lived charge carriers is decreased and increased, respectively.
3. Morphology and Structure Design of g-C3N4
3.1. Hard and Soft Template Approach
Apart from regulating the synthesis parameters, introducing nano-templates and nano-casting with different morphology and ordered porosity on the basis of bulk g-C3N4 is another promising method to change the morphology and structural characteristics of g-C3N4 structure and the interlayer interaction. As a matter of fact, researchers have effectively designed controllable nanostructures for g-C3N4 through hard template or soft template methods, such as porous g-C3N4, one-dimensional nanostructures, hollow g-C3N4 nanospheres, etc [11,77,78,79,80,81,82,83,84,85]. It has been proven that the porosity, structure, morphology, surface area and size can be easily controlled by adjusting the appropriate template. Moreover, the larger surface area and more active sites are generally more favorable for photocatalytic applications of g-C3N4.
The hard template method is almost identical to the traditional casting process and is one of the most common techniques for developing nanostructured g-C3N4 materials. In this way, the various structures and geometries of g-C3N4 can be designed using hard templates as needed, and their length scales are usually around nanometers and microns. The most typical structure-oriented agent is a silica template with a controllable nanostructure. The early study on the mesoporous g-C3N4 synthesized using cyanamide as a precursor and silica nanoparticles with a size of 12 nm as a template was reported by Goettmann et al. [40]. The results show that the silica nanoparticles can be uniformly dispersed in the cyanamide monomer, which is due to the appropriate surface interaction between the silica surface and the amine and aromatic nitrogen groups.
It can be seen that during the synthesis of g-C3N4 through hard templates, extremely dangerous, toxic and expensive fluorine-containing etchers (such as HF and NH4HF2) are used to remove the sacrificial templates. This greatly limits the practical application of the method in large-scale industrial processes. Therefore, apart from the hard template synthesis method of g-C3N4, the relatively “environmentally friendly” soft template process can not only change the morphology and structure of g-C3N4 through the selection of multiple soft templates, but also simplify the synthesis route of g-C3N4 [88,89].
3.2. Supramolecular Preorganization Approach
In contrast to the previously discussed hard and soft template synthesis approaches, molecular self-assembly is a self-templating approach (namely supramolecular preorganization approach) in which molecules spontaneously form a stable g-C3N4 structure from non-covalent bonds under equilibrium conditions in the absence of an external template [80,92,93,94]. Recently, supramolecular preassembly of triazine molecules has become an interesting method to regulate the structural, textural, optical, and electronic features of g-C3N4, thus affecting its photocatalytic activity [95,96,97,98]. For example, nanostructured g-C3N4 materials can be developed by supramolecular preorganization of melamine precursors to triazine derivatives to form hydrogen bond molecular assemblies, i.e., melamine–cyanuric acid, melamine–trithiocyanuric acid mixtures or their derivatives [99,100,101].
It can be seen that the combination of two or more monomers in different solvents can form supramolecular complexes. These supramolecular complexes are usually linked by hydrogen bonds. Therefore, it is expected that the addition of new monomers to hydrogen-bonded supramolecular complexes as “terminators” will be an attractive technique to further adjust the morphology, photophysical properties, and electronic band structure of g-C3N4.
3.3. Template-Free Approach
Compared with the hard and soft template synthesis approaches, the template-free approach has unique advantages, such as no need for various high-cost and dangerous templates containing fluorine, and no residue of any template components. Indeed, many studies have proven that g-C3N4 nanostructure designs with a variety of morphologies and desired sizes, such as nanorods, quantum dots, microspheres, nanofibers, etc., can also be achieved using a template-free approach. Bai et al. reported that the transformation of g-C3N4 from nanoplates to nanorods was realized by a simple reflux method [103].
Additionally, Wang et al. described a facile and generally feasible method to synthesize nanotube-type g-C3N4 by directly heating melamine packed in an appropriate compact degree without templates [104]. A certain amount of melamine was placed into a semi-closed alumina crucible followed by consecutively shaking the crucible using a vibrator at a fast rate to achieve a moderately compact packing degree. This process is very crucial for the synthesis of nanotube-type g-C3N4. TEM images show that the wall thickness of the nanotubes in the bulk phase is about 15 ± 2 nm, while the inner diameter is about 18 ± 2 nm. During the pyrolysis process, melamine releases NH3 gas, which passes through the stacked melamine layers to form rolled g-C3N4 nanosheets.
4. Exfoliation of Bulk g-C3N4
Although the specific surface area of the monolayer g-C3N4 is theoretically large, the specific surface area of the block is very low indeed, usually less than 10 m2 g−1, due to the stacking of g-C3N4 layers [105]. Therefore, delaminating g-C3N4 into several layers is a promising way to improve photocatalytic performance and produce more interesting surface, optical and electronic properties [106,107,108,109]. There are many methods of exfoliating g-C3N4, such as ultrasonication-assisted liquid exfoliation, the liquid ammonia-assisted lithiation and the post-thermal oxidation etching route [17,108,109,110,111,112,113,114,115].
Liquid exfoliation is simple and convenient, and has gradually become the most commonly used exfoliation method by most researchers. Yang et al. demonstrated the synthesis of free-standing g-C3N4 nanosheets by liquid phase exfoliation [110]. The method uses g-C3N4 powder as a starting material and various organic solvents (such as isopropanol (IPA), N-methyl-pyrrolidone (NMP), acetone, and ethanol) as dispersing media.
5. Doping of g-C3N4
It is well-known that g-C3N4 is a metal-free n-type semiconductor. Due to the high ionization energy and high electronegativity of metal-free semiconductors, it is easy for them to form covalent bonds with other compounds by obtaining electrons during the reaction. In order to maintain this unique advantage of metal-free semiconductors, researchers have implemented a series of non-metal doping g-C3N4, including oxygen, phosphorus, sulfur, carbon, halogen, nitrogen and boron [116,117,118,119,120,121]. For instance, O-doping is a facile method to improve the photocatalytic ability of g-C3N4. Zeng et al. synthesized one-dimensional porous architectural g-C3N4 nanorods by direct calcination of hydrous melamine nanofibers precipitated from an aqueous solution of melamine [122]. The porous structure increases the specific surface area, enhances the light absorption capacity and improves the catalytic reaction rate. At the same time, doping oxygen atoms into the g-C3N4 matrix breaks the symmetry of the pristine structure, making more efficient separation of electron/hole pairs. In general, non-metallic doping usually changes the surface morphology and structure of g-C3N4, thereby affecting the light absorption efficiency and regulating the catalytic efficiency.
6. Applications of g-C3N4
Due to its moderate energy gap, excellent electronic properties, rich functional groups and surface defects, g-C3N4 can be widely used in environmental treatment and pollutant degradation, including water splitting, hydrogen generation, CO2 conversion and organic pollutants degradation [129,130,131,132].
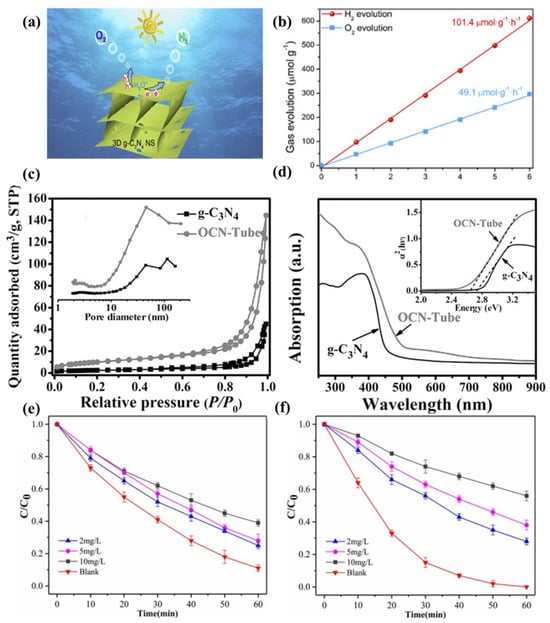
As reported, the pristine g-C3N4 has limitations such as small specific surface area and fast charge recombination rate, which leads to a low water splitting ability of g-C3N4. To solve this problem, Chen et al. improved the water splitting capacity via adjusting the dimension of g-C3N4 [133]. As shown in Figure 6a,b, they demonstrated that the evolution rates of H2 and O2 of three-dimensional porous g-C3N4 in visible light are significantly higher than those of the pristine g-C3N4, reaching 101.4 and 49.1 μmol g−1 h−1, respectively. Fu et al. reported oxygen-doped g-C3N4 [134]. As exhibited in Figure 6c-d, the O-doped g-C3N4 has a narrower band gap and greater CO2 affinity, which significantly improves the photogenerated carrier separation efficiency and CO2 conversion ability. In addition, a lot of efforts have been made to enhance its photocatalytic activity to improve its pollutant degradation ability. As shown in Figure 6e-f, Dou et al. reported that mesoporous g-C3N4 has a strong ability to remove antibiotics under visible light [135], which is mainly due to the porous structure that improved the utilization of light.

Figure 6. (a) Water splitting for H2 and O2 Evolution. (b) Time−dependent overall water splitting over 3D g-C3N4 [133]. (c) N2 adsorption–desorption isotherms and corresponding pore size distribution curves. (d) UV–vis diffuse reflectance spectra [134]. The effects of humic acid on (e) amoxicillin and (f) cefotaxime photodegradation by mesoporous carbon nitride (initial pH = 7) [135].
7. Conclusions
In summary, due to the merits of low cost, high stability and visible light response, g-C3N4 is one of the most promising photocatalytic materials to replace TiO2. As summarized above, the appropriate reaction temperature and duration of the condensation process are beneficial to improve the crystallinity of g-C3N4. Various desirable nanostructures of g-C3N4 can be constructed via hard and soft template approaches, supramolecular preorganization approach, and template-free approach. Liquid exfoliation of bulk g-C3N4 has becoming the most facile and promising method to improve the surface area of g-C3N4.
Therefore, in order to synthesize the ideal g-C3N4 with high photocatalytic efficiency, it is necessary to pay attention to the following crucial elements: (i) Controlling the corresponding reaction temperature and reaction time according to the selected precursor material; (ii) Controlling the C/N ratio close to 0.75 and the band gap to 2.7 eV; (iii) Extending the specific surface area by selecting suitable nanostructure design approaches.
This entry is adapted from the peer-reviewed paper 10.3390/catal13111402
This entry is offline, you can click here to edit this entry!
