The intestinal microbiota contributes to maintaining the integrity of the intestinal barrier, preventing the formation of a “leaky” intestine. On the contrary, a change in the composition of the microbiota can act as a significant link in the pathogenesis of gluten intolerance and exacerbate the course of the disease. The possibility of modulating the composition of the microbiota by prescribing probiotic preparations is being considered. The effectiveness of the use of probiotics containing Lactobacillus and Bifidobacterium bacteria in experimental and clinical studies as a preventive and therapeutic agent has been documented.
1. Introduction
Celiac disease’s etiology involves a reaction of the body to a certain component of food, namely gluten, through T cells. There are also numerous other variables contributing to the onset of this disease [
27]. As a result, immuno-conditioned enteropathy develops. A feature of the intestinal lesion is the reversible atrophy of its villi and hyperplasia of crypts with the exclusion of a trigger agent. The prevalence of celiac disease according to various data ranges from 1:100 to 1:300 in the world; the ratio of women to men is 2:1 and it is higher in children than in adults (0.9% versus 0.5%) [
28,
29,
30,
31,
32]. Celiac disease is more common in girls and can occur after eating gluten-containing foods at any age, including infancy [
33]. Due to the nonspecificity of symptoms and the unclear clinical picture, the diagnosis is often missed even in developed countries [
28]. Of course, in underdeveloped countries, diagnosis is even worse, which is a consequence of limited access to diagnostic tests and lack of experience [
34].
Taking into account the clinical picture and the results of laboratory studies, two forms of the disease are distinguished: symptomatic (manifest) and asymptomatic (latent) [
27,
35,
36]. The main manifestations of celiac disease are characterized by the presence of gastrointestinal symptoms (persistent diarrhea, flatulence, abdominal pain, nausea and others) and/or extraintestinal manifestations (osteoporosis, anemia, infertility, neurological symptoms and others). In the case of the latent form, the diagnosis is established during screening examinations. The first symptoms of the disease usually appear in childhood, 1.5–2 months after the start of consumption of gluten-containing products [
37].
Gluten, as the main initiating factor of the disease, is a protein contained in cereal grains, which, along with starch, is present in their composition and consists of glutelins and prolamins. It has been confirmed that the amino acid structure of glutelin and prolamin in wheat, barley and rye is the most immunogenic for patients suffering from celiac disease [
38,
39]. Apparently, the change in gluten sensitivity is formed as a result of the influence of many different factors. On one hand, there is information that celiac disease develops in the presence of a genetic predisposition associated with certain human leukocyte antigen (HLA) genes of the second type, known as DQ2 and DQ8 [
40,
41]. However, this genotype is common and occurs in about 35% of the population, though only 3% of these people have this disease. Autoantigen tissue transglutaminase (TG) is also involved in the pathogenesis of celiac disease [
42]. This indicates a possible and compelling role of other factors in the development of celiac disease, including disorders in the composition of the intestinal microbiota and increased permeability of the intestinal wall [
43,
44,
45,
46]. Given that celiac disease is characterized by inflammation that occurs in the small intestine, it can be assumed that the local microenvironment, which is significantly influenced by the microbiota, plays a decisive role in the pathogenesis of the disease and the violation of tolerance to dietary gluten. Intestinal microflora can influence the development of celiac disease through various mechanisms [
47]. The microbiota, due to the peptidases secreted by it, is capable of both forming immunogenic peptides and eliminating immunogenic peptides that are not cleaved by intestinal enzymes. Some bacteria are able to express epitopes with a structure similar to gliadin, thereby triggering an immune response in the host [
24]. At the same time, the microbiota can influence the formation of antigen by modulating the digestive process, generating either immunogenic or tolerant gluten peptides. In addition, the microbiome can directly affect intestinal permeability. Intestinal microbes are also involved in the regulation of immune responses, producing peptides, metabolites and cytokines that have both proinflammatory and anti-inflammatory properties [
48].
2. The Role of the Gut Microbiota in the Pathogenesis of Celiac Disease
2.1. Genetically Susceptible
Several studies have revealed that infants with HLA-DQ2 and HLA-DQ8 genotypes, as well as first-degree relatives of patients with celiac disease, have a change in the composition of bacteria in the intestinal microbiota [
49]. This change is expressed in an increase in the representatives of the
Firmicutes and
Proteobacteria groups, as well as a decrease in the number of
Actinobacteria. The data obtained suggest that the HLA genotype is associated with certain changes in the composition of the intestinal microflora, which are characteristic of patients with celiac disease and their close relatives [
50,
51,
52,
53,
54,
55].
Several studies indicate a higher incidence of celiac disease among children born by caesarean section, as well as among those who are artificially fed and have also received antibacterial drugs in the first year of life [
51,
56,
57,
58,
59,
60,
61,
62]. These facts are known to affect the composition of the intestinal microbiota, which confirms the hypothesis of its significant role in the pathogenesis of this disease.
Interesting results were obtained in the study of breast milk samples from mothers suffering from celiac disease. These mothers were found to have lower levels of interleukin 12p70, transforming growth factor-β1 and secretory immunoglobulin A (sIgA, secretory immunoglobulin A). A decrease in the amount of
Bifidobacterium and
Bacteroides fragilis in breast milk was also observed. This study confirms the hypothesis that a decrease in the level of immunoprotective molecules and certain types of
Bifidobacterium can reduce the protective properties that breastfeeding usually provides, and, consequently, increase the risk of a child developing celiac disease [
62,
63].
2.2. The Composition of the Microbiota
Comparison of the composition and functional activity of bacterial communities in patients with celiac disease and healthy volunteers makes it possible to better understand the contribution of microorganisms to the development of the disease. In the case of celiac disease, changes in the composition of the microbiota are observed: the proportion of
Bacteroidetes and
Proteobacteria increases, and the content of
Lactobacillus,
Bifidobacterium and
Faecalibacterium prausnitzii decreases [
50,
64,
65,
66,
67,
68,
69]. Differences in the composition of
Lactobacillus were found in children with celiac disease (predominance of
L. curvatus) as compared to healthy children (
L. casei,
L. paracasei,
L. rhamnosus,
L. zeae). The composition of
Bifidobacteria also changes, with a significant decrease (and even complete absence) of
Bifidobacterium longum in patients with celiac disease [
70,
71]. Literature sources indicate an increase in the composition of the intestinal microbiota of
Bacteroides vulgatus and
Bacteroides fragilis, which is important because of their gliadin-specific protease activity. It is also noted that virulence and proinflammatory activity are enhanced in some microorganisms, including
Enterobacteriaceae and
E. coli isolated from patients [
72,
73].
2.3. Digestive Proteases
The function of human digestive proteases in the processing of gluten proteins has been extensively researched. The exceptional resistance of glutelin and prolamin to the action of proteolytic enzymes is recognized. This partially decomposed process creates peptides with enterotoxic properties. It is assumed that the presence of a “predisposition” to celiac disease allows these peptides to penetrate the mucous layer, causing a specific inflammatory reaction [
28,
29,
30,
41,
75,
76,
77].
3. Changes in Intestinal Permeability in Celiac Disease
3.1. The Intestinal Mucosal–Epithelial Barrier
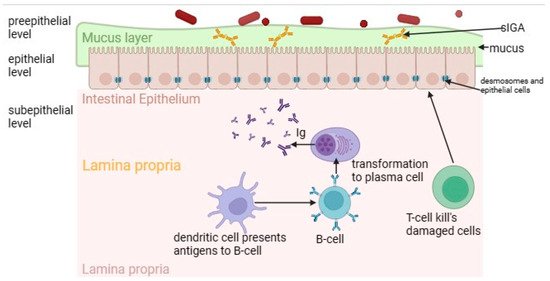
Intestinal permeability plays a key role in the pathogenesis of gluten enteropathy, which is normally regulated by a multilevel mucosal–epithelial barrier (
Figure 1) [
80]. The pre-epithelial level of the small intestine is represented by a dense layer of mucus providing a barrier function, which includes antimicrobial peptides, sIgA and glycoproteins [
81,
82]. The gel-forming mucin MUC2, which is the primary component of mucus in the small intestine, provides protection by inhibiting the adherence of pathogenic bacteria to the intestinal epithelium. While maintaining the balance of microbiota and mucus composition, including normal MUC2 expression, selective impermeability of the pre-epithelial level is maintained [
82,
83,
84].
Figure 1. The intestinal mucosal–epithelial barrier in normal circumstances. It consists of pre-epithelial, epithelial and subepithelial levels. The pre-epithelial level is represented by a mucus layer. The epithelial level is represented by enterocytes, which are also closely connected by desmosomes. The subepithelial level includes lamina propria with immune cells.
At the next level—epithelial—there are enterocytes closely connected by a connective complex that maintains the structural integrity of the barrier and regulates the paracellular permeability of the small intestine. This complex includes dense contacts such as tight junction proteins (TJ proteins); transmembrane proteins such as occludin and claudins; peripheral membrane proteins, for example, actin-filament-binding scaffold proteins (ZOs); adhesive molecules (junctional adhesion molecule (JAM), connective adhesion molecule); adhesive contacts (E-cadherin and β-catenin proteins); slit contacts (connexin proteins); and desmosomes [
85].
Finally, the deepest layer—the subepithelial layer—is represented by its own plate of the mucous membrane. Thanks to the cells of the immune system (T-lymphocytes, B-lymphocytes, macrophages, dendritic cells), this layer provides immunological protection of the intestinal barrier [
86]. The intestinal barrier is a dynamic structure that reacts to the effects of various triggers.
3.2. The “Leaky” Intestine
It is proved that the violation of the integrity of the epithelial barrier is one of the main etiological factors associated with a number of diseases of the gastrointestinal tract, obesity and diabetes [
88]. The violation of the integrity of the mucosal–epithelial barrier in celiac disease is indisputable. However, there is still a debate in the scientific community about whether altered intestinal permeability is the cause or a consequence of immune-mediated reactions. It is obvious that the development of a “leaky” intestine aggravates the nature of the disease [
89,
90]. The increase in intestinal permeability is due to the interaction of gluten and changes in the microbiota. The composition of the intestinal microbiota influences all aspects of the mucosal–epithelial barrier. In addition, changes in the qualitative and quantitative composition of the intestinal microflora serve as a causal factor in activating the immune system of the intestinal wall with the development of subclinical inflammation, changes in motor function and the development of visceral hypersensitivity, which ultimately leads to disruption of the interaction of the brain–gut–microbiota axis [
91,
92].
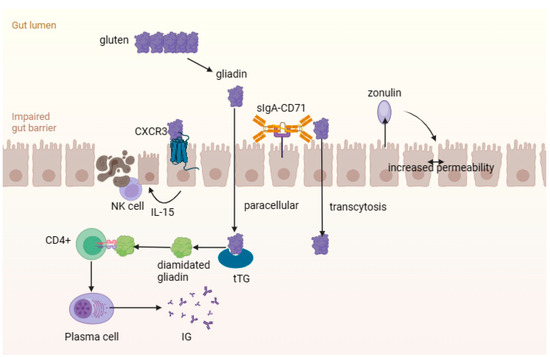
How can celiac disease lead to the development of the “flowing” intestinal state? After gluten enters the body, it undergoes decomposition with the aid of intestinal proteases and endopeptidases. Binding of gliadin peptides to the type 3 chemokine receptor (CXCR3) on epithelial cells of the small intestine causes the release of a large amount of zonulin via a signaling pathway dependent on the MyD88 protein (
Figure 2). Zonulin, which plays an important role in regulating the permeability of the intestinal wall, activates the actin components of the cytoskeleton of cells associated with zonulin proteins, which leads to an increase in the gaps between cells [
41,
89,
96,
97]. Zonulin also enhances intestinal wall permeability by activating the epidermal growth factor receptor (EGFR) via a protease-2 (PAR-2) activated receptor [
98]. Disruption of the function of intercellular junctions and the emergence of a “paracellular” pathway for toxic gliadin peptides in the intrinsic plate of the mucous membrane is also associated with an imbalance in the expression of proteins forming a binding structure between enterocytes (decreased expression of E-cadherin, β-catenin and claudins 3 and 4, and increased expression of claudin 2) and cytoskeletal rearrangement. The penetration of gliadin into its own plate of the mucous membrane of the small intestine can also be carried out through the sIgA-CD71 complex using transcytosis [
99,
100,
101].
Figure 2. The intestinal mucosal–epithelial barrier in celiac disease. Gluten undergoes decomposition to gliadin peptides, which bind with the type 3 chemokine receptor (CXCR3) and cause the release of zonulin. This increases the gaps between cells and penetration of gliadin into lamina propria. Gliadin peptides contribute to the activation of the innate immune response.
4. Effects of Probiotics
4.1. Taking Probiotics
Lactobacillus and
Bifidobacterium are typical components of commercial probiotic products. Less popular probiotics are usually based on
Escherichia or
Saccharomyces. The main conclusion that can be drawn on the basis of available clinical studies is that the effectiveness of one strain of a microorganism cannot be extrapolated to another strain of this microorganism [
109]. The evidence base of the clinical efficacy of probiotics is presented with systematic reviews and meta-analyses of randomized controlled trials. Mosafarybazargany et al. noted that probiotics might alleviate gastrointestinal symptoms, especially in highly symptomatic patients, and improve the immune response in celiac disease and celiac disease autoimmunity patients [
110].
The effects of probiotic microorganisms in celiac disease are studied both in experimental and clinical studies. The beneficial effects of probiotics on the diversity of the intestinal microflora and its main metabolites, including short-chain fatty acids, as well as translocation to other organs of the normoflora are reported [
111]. The administration of
Lactobacillus casei to laboratory animals for 35 days resulted in complete restoration of the villi of the small intestine, reduced weight loss, normalization of basal TNF-α levels and no changes in CD25+ cells and IL-2 levels [
112].
4.2. The Use of Probiotics for the Breakdown of Gluten in Food
At the moment, various Lactobacilli species capable of decomposing gluten are known—these are
L. ruminus,
L. john donne,
L. amylovorus,
L. salivarius,
L. alimentaris,
L. brevis,
L. sanfranciscenis and
L. hilgardi. Studies have shown that if these Lactobacilli species are added to the starter culture for the production of wheat bread, the endopeptidases of these microorganisms are able to decompose gluten peptides. This, in turn, leads to a decrease in the concentration of gluten to levels below 10 ppm (the threshold of gluten-free food) and a decrease in the immunotoxicity of its peptides. In patients suffering from celiac disease and consuming such wheat bread produced with Lactobacilli, there was no worsening of symptoms, increased intestinal permeability or changes in serological markers [
121].
5. Conclusions
In sum, scientific studies confirm the changes in the composition of the microbiota in celiac disease, which supports the hypothesis of changes in intestinal bacterial communities. These changes affect the pathogenesis of the disease. It is noted that the intestinal microbiota may have a protective effect on the development of celiac disease. The intestinal microbiota contributes to maintaining the intestinal barrier’s integrity, preventing “leaky” intestine formation. The prescription of probiotics for the treatment and prevention of celiac disease shows encouraging results. The effectiveness of the use of probiotics containing Lactobacillus and Bifidobacterium bacteria in experimental and clinical studies as a preventive and therapeutic agent has been documented. Preliminary results have proven that the addition of probiotics to a gluten-free diet reduces intestinal hyperpermeability and improves the immune response of the intestine, restoring the normal architecture of the villi.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11122848