1. Biochar: Feedstock, Synthesis Methods, and Properties
Biochar mainly comprises carbon (~60–90%), although it may also contain oxygen, hydrogen, and inorganic ash depending on the source biomass [
67]. Biochar conversion is considered more environmentally benign than coal combustion, as biomass is carbon neutral [
68]. Generally, biochar has a high surface area (above 100 m
2/g), which depends on the raw material and the synthesis conditions [
69]. As a result, biochar can be used in a variety of nonfuel applications, such as chemical adsorption (e.g., water treatment [
21,
70]) and carbon storage [
68]. In addition, this carbonaceous material has also been used as a soil fertilizer [
71,
72].
1.1. Differences between Biochar, Activated Carbon and Charcoal
The carbon family involves interesting materials, such as biochar, charcoal, and activated carbon. These carbonaceous materials share the essence and origin, which is carbon. The most significant distinction is their synthesis methods, conditions, and applications. On the one hand, coal results from coalification, i.e., a geological process involving biomass conversion with water and sediments. Peat and lignite are intermediate stages of this process [
73].
On the other hand, charcoal, biochar, and activated carbon are products of thermochemical processes, and they are defined as pyrogenic carbonaceous materials (PCM) [
24]. Biochar and activated carbon are frequently used in agriculture for environmental remediation, such as filtering and purification. Meanwhile, charcoal is used for heating and cooking [
74,
75]. These materials’ physical and chemical properties are similar since they share the same carbonaceous origin. However, they have singular properties that distinguish them.
Table 1 describes the marked difference between these carbonaceous materials and the main characteristics that define them and differ from each other.
Biochar is a member of the carbon family that, when mixed with other species, produces new hybridized nanomaterial biochar-based materials [
76]. They have novel physicochemical properties and are highly effective for degrading water pollutants through adsorption, heterogeneous photocatalysis, and advanced oxidation processes. Braghiroli et al. [
77] have reported a high sorption capacity of phenols and chemical intermediates with the use of activated biochar and other biochar-based materials for treating phenolic compounds, such as phenol, bisphenol A, p-nitrophenol, and pentachlorophenol, are toxic to health and the environment [
78].
Table 1. Description of the most influential carbonaceous materials.
Compared to pure nano-photocatalysts, biochar-supported catalysts have larger surface areas, are more porous, have higher catalytic capacities, and are more stable [
82]. Biochar may support hosting different catalytic nanoparticles because of its unique surface features, readily modifiable functional groups, chemical stability, and electrical conductivity [
83,
84].
1.2. Feedstock for Biochar Production
Biomass is living or once-living organic matter that can serve as a versatile renewable source for environmental and energy applications (e.g., electricity generation, heat provision) and for the production of many types of biofuels, compost, pharmaceutical products, other chemicals, and biomaterials, like biochar. Almost all organic materials (such as tree bark, nut shells, crop residues, and manure) can be used as feedstock for biochar using appropriate equipment [
85,
86,
87]. Biomass as an initial resource can come from animal, vegetable, or human-generated waste, such as industrial or municipal waste (sewage) [
66,
88]. Biochar-based materials can have different characteristics and properties depending on the biomass used as feedstock, which, in turn, will allow the carbonaceous material to be used in specific applications.
Table 2 shows the most common feedstock used to produce biochar, which can be any organic matter, from plant materials to industrial waste.
Table 2. Commonly used feedstock for biochar production [
89,
90,
91,
92].
Biochar is commonly produced from vegetal residues called cellulosic biomass, such as firewood or rice residues. In recent years, other raw materials have been studied to produce biochar, such as algae, food waste, manure, and animal tissue [
63,
90,
91], obtaining interesting results regarding its physical-chemical properties and applications. On the other hand, raw materials with a high biomass content, such as sewage and municipal solid waste (MSW), cannot be considered a suitable feedstock for biochar production since they may include contaminating components that can affect the biochar performance for soil or water treatments [
93].
1.3. Synthesis Methods Used to Prepare Biochar
Biomass can be transformed using thermochemical conversion processes, like pyrolysis or HTC treatment, to produce biogas, liquid fuels (e.g., bio-oil), and solid materials, such as biochar [
8,
63]. Biomass valorization can be conducted through basic processes (e.g., pyrolysis, gasification, torrefaction, anaerobic digestion, or combustion), in which the organic matter can be transformed into heat, electricity, or by-products, like biochar-based materials [
94]. Thermochemical conversion encompasses the degradation of biomass structure in either an oxygenic or anoxygenic atmosphere at high temperatures [
95]. Biochar production begins from the initial conversion of biomass through thermochemical processes until a carbonaceous material with desired physicochemical properties is obtained. The operating principles, synthesis conditions, and their effect on biochar production for the most common thermochemical conversion processes are detailed below:
1.3.1. Pyrolysis
In the pyrolysis process, the biomass source, which was previously mentioned, is subjected to a thermal treatment to produce biochar and other by-products. Depending on the operating conditions, biogas, liquid bio-oil, and biochar can be generated during this process [
96]. It is essential to note that biomass must be previously dried and ground to obtain a carbonaceous material of high quality and yield. The heating process is conducted at high temperatures (400–800 °C) without oxygen and allows biomass conversion into by-products for several applications, such as energy and environmental remediation. The by-products can be used as energy or residual heat to contribute to the pyrolysis or thermal treatment of the raw material. This thermal process releases the lowest percentage of carbon back into the atmosphere [
58,
71,
97]. According to the literature, there are two types of pyrolysis: slow and fast pyrolysis, which depend on the temperature conditions and the heating rate [
98]. The slow pyrolysis is conducted at temperatures ranging from 250 to 600 °C using heating rates of 1–10 °C/min [
90,
99]. While fast pyrolysis is based on the thermal conversion of biomass at temperatures above 600 °C, using heating rates higher than 50 °C/min [
100]. The concentration and the physicochemical properties of the products formed (e.g., biogas, bio-oil, and biochar) can vary depending on the type of pyrolysis. Thus, during the slow pyrolysis of biomass, a large amount of biochar can be produced, generating low concentrations of gases and liquids with a high content of highly contaminant volatile organic compounds (VOCs) [
101].
On the other hand, fast pyrolysis is mainly used to produce a high concentration of liquids (e.g., biofuel) with better physicochemical quality than those produced by slow pyrolysis, achieving a lower VOC content and a higher concentration of log-chain hydrocarbons [
102]. Both types of pyrolysis can be used to produce biochar. However, the properties (e.g., carbon content, density, water retention capacity, functional groups, surface area) and applications of the carbonaceous materials will be different. Slow pyrolysis can be the best way to obtain water and soil remediation biochar. Meanwhile, fast pyrolysis can be the best route to produce biochar as fuel or precursor to other materials [
101,
103].
The pyrolysis of biomass modifies the size and arrangement of the carbonaceous structures, enhancing the physicochemical properties of the products obtained during the process [
104]. In general, this impact becomes more robust at higher treatment temperatures. To obtain a higher biochar production yield, the temperature interval for pyrolysis should be around 400–800 °C [
105,
106]. Lua et al. [
81] reported that by raising the pyrolysis temperature from 250 to 500 °C, the specific surface area can increase from 170 to 480 m
2/g, which has been related to the increased evolution of volatile matter in pistachio nut shells, resulting in an improved pore growth at the biochar surface, reaching total pore volume values of 0.47 cm
3/g (at 500 °C), which were much higher than those obtained at a pyrolysis temperature of 250 °C (0.193 cm
3/g). This has been related to elevated temperatures supplying activation energy, which can favor conversion reactions, resulting in higher degrees of order in the carbonaceous structures [
107].
1.3.2. Torrefaction
Similarly to pyrolysis, torrefaction is a thermochemical process based on biomass conversion into value-added products, e.g., biochar, biogas, and bio-oil [
108]. However, this process differs from pyrolysis in operating conditions and formed product types. Torrefaction is a thermal process based on biomass dehydration, carbonization, and caramelization at relatively low temperatures (i.e., 200 to 300 °C) without oxygen [
58]. Biochar is the only product generated during this process. However, the physicochemical properties (e.g., structural characteristics) of the carbonaceous material produced are inferior to those of pyrolysis [
62,
91].
1.3.3. Hydrothermal Carbonization
Hydrothermal carbonization (HTC) is a thermochemical technology for processing biomass with high moisture content in a hot compressed water system [
14]. The main product of HTC is hydrochar, a type of biochar produced in this way. Apart from this carbonaceous material, aqueous (nutrient-rich) and gas phases (mainly CO
2) can be produced depending on the operating conditions [
109]. The carbon-rich hydrochar can be employed as fuel, coal substitute, gasification feedstock, soil additive for nutrient enrichment, or as an adsorbent or precursor of activated carbon [
110]. The advantage of the HTC process is that biomass may be transformed into carbonaceous solids without an energy-intensive drying procedure or an anoxygenic atmosphere. Likewise, toxic chemical compounds and residual micropollutants are also avoided during HTC [
111].
As mentioned below, HTC is a thermochemical process that uses heat to transform wet biomass feedstock into hydrochar. HTC is performed in a reactor at temperatures ranging from 120 to 300 °C under autogenous (self-generated) pressure or under pressure (2–6 MPa) with feedstock residence periods ranging from 0.5 to 8 h [
112,
113,
114]. HTC offers a key advantage over other high-temperature thermochemical conversion processes (e.g., pyrolysis) because it is possible to use wet waste without a pre-drying process [
19,
115]. HTC may use a variety of feedstock, including aquatic biomass, agricultural waste, and industrial and animal waste [
11]. Water is a favorable medium for heat transfer in HTC. However, there may be some mass transfer restrictions if the particle size variability in the feedstock is too large (above 2 cm) and the reaction time is too short (less than 30 min) [
19]. As a result, particle size should be constant to provide uniform heat and mass transfer. On the other hand, the aqueous slurry needs to be centrifuged or filtered to separate the process water and particulates (wet cake). Biomass conversion processes mainly depend on the feedstock, the desired final product, and its corresponding use.
Table 3 shows a summary of the typical thermochemical conversion processes, temperature conditions, and the products obtained in each of them.
Table 3. Thermochemical conversion processes to obtain biochar used in different fields [
9,
116,
117].
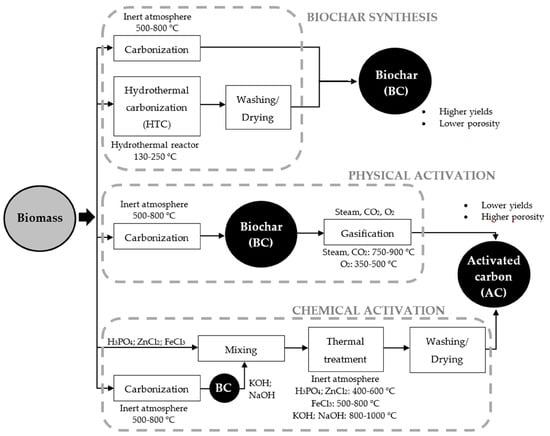
1.4. Methods of Biochar Activation and Modification
Physical and chemical activation methods can enhance biochar properties, such as impregnation or adding dopants or additives in the carbonaceous structure. Physical activation is accomplished by processing biochar with oxidizing agents, mostly steam or carbon dioxide, at temperatures ranging from 500 to 1000 °C. Water is a smaller molecule than carbon dioxide, which favors its penetration into the biochar pores [
19,
118], enhancing its morphological properties, like surface area and porosity [
119].
Figure 1 shows some routes of physical and chemical activation of biochar, as well as the chemical compounds and thermal conditions used to enhance its physicochemical properties. Notably, the porosity, surface chemistry, and yields of carbon-based adsorbents produced significantly depend on the biomass composition of feedstock and the synthesis conditions [
120].
Figure 1. Activation routes of biochar and activated carbon. (Reprinted from [
120]). © 2018 by the authors.
Many modification methods (e.g., chemical, physical, and biological routes) have been studied to improve the properties of biochar used for environmental purposes [
63]. The widely used method has been the chemical alteration. Acid modification, alkalinity modification, oxidizing agent modification, metal salts, or oxidizing agent modification are the most common. In contrast, steam and gas purging have been the most common types of physical modification [
19]. Physical activation of biochar using steam and chemical activation with acidic and alkaline solutions is usually performed after pyrolysis. However, remarkable results have been seen when chemical activation is performed before pyrolysis [
122].
Interesting and novel physicochemical activation methods of biochar seek to improve functional stability, and these can be based on its modification with other species. In this sense, biochar-based composites can be prepared by impregnating or coating their surface with metal oxides, clays, carbonaceous structures (e.g., graphene oxide or carbon nanotubes), complex organic compounds, such as chitosan, among others [
32,
123,
124].
1.4.1. Physical Activation
Physical activation enhances the surface pores of biochar and can also modify its chemical properties (e.g., surface functional groups, hydrophobicity, and polarity) [
125]. Steam activation enhances the surface area and porosity of biochar [
126]. Zhang et al. [
127] have reported sludge-based pyrolysis to produce biochar, which was activated using a physical activator (CO
2) to enhance its adsorption capacity of Pb
2+ from an aqueous solution. The results revealed that the physical activation with CO
2 enhanced the specific surface area by more than ten times, and its Pb
2+ adsorption capacity increased from 7.6 mg/g to 22.4 mg/g [
127]. The biochar activation with CO
2 aided in the introduction of oxygen-containing functional groups. On the other hand, biochar activation with CH
3COOK also enhanced the pore structure of sludge-based biochar, increasing its surface area more than ten times, from 81 m
2/g to 908 m
2/g, reaching a Pb
2+ adsorption capacity of 47.6 mg/g [
127].
During physical activation, biochar is exposed to a required amount of oxidizing agents, such as steam, ozone, carbon dioxide, or air, at temperatures typically above 500 °C [
128]. These oxidizing chemicals enter the biochar structure and gasify the carbon atoms, opening and expanding previously inaccessible pores [
129]. This type of activation can produce a biochar with larger surface areas and generate a large amount of surface oxygen functional groups, which frequently serve as active adsorption sites for pollutant removal [
129].
Another physical process, like steam activation, is gas purging, in which gases (such as carbon dioxide) are mixed with the accessible amorphous carbon at the biochar surface in a restricted oxygen environment to produce carbon monoxide [
130]. Moreover, carbon monoxide formation can increase the biochar surface area, improving its microporous structure and pore volume [
27].
1.4.2. Chemical Activation
The most typical route for modifying the type and number of functional groups at the biochar surface is chemical activation, which involves doping a chemical agent into its structure. In this process, the raw material (i.e., biomass) is impregnated with a chemical agent, and the combination is subsequently thermally treated to obtain a biochar-doped material [
129]. During the process, the chemical agent can act as an activator, which favors sample dehydration and prevents the generation of tar and volatile chemicals, thus increasing the yield of the carbonization process [
131]. In addition, these activators can be used to increase the biochar-specific surface and pore volume and generate functional groups in its structure.
Depending on the final purpose of the carbonaceous material, acid or alkali activation can be employed. When soil amendment or water purification (heavy metal or colorant adsorption) is performed, acidic activation is preferred over alkali activation [
132]. Alkali activation is more related to producing materials for energy storage [
133,
134] or electrochemical processes because of their high capacitances [
132,
133]. The impregnation of specific elements or promoters to increase biochar adsorption capacity has been widely reported for water purification.
As previously mentioned, the most common activators are alkalis (KOH, NaOH, and ZnCl
2) [
130] and acids (citric, nitric, sulfuric, and phosphoric) [
119,
135,
136]. H
3PO
4 is commonly used as an activator because it can promote the bond breakage processes while maintaining the internal pore structure [
137]. The distribution of chemical agents in the precursor before carbonization plays a vital role in the final product’s porosity improvement and functionality [
138]. According to Fierro et al. [
139], the effect of the added quantity of phosphoric acid for the activation of carbon derived from rice straw is essential to increase its yield until a certain quantity. When they used a H
3PO
4: biomass ratio equal to 1 (ranging from 0 to 1.6), the carbon yield increased by up to 10%.
Moreover, when the ratio was more significant than 1, an increase in the percentage of the carbonization yield was not observed. However, the specific surface area of the carbonaceous material increased from 520 to 786 m
2/g. In this work, the volume of the pores was highly variable, and no tendency to deformation was observed. Likewise, Zakaria et al. [
140] have reported that the effect of phosphoric acid to obtain mangrove-based activated carbon (with the H
3PO
4: precursor ratios of 3, 4, and 5) on its production yield and surface characteristics are also notable. They observed a gradual decrease in the yield of activated carbon (45–41%) as the ratio increased from 3 to 5. Other authors have also reported this fact [
141,
142,
143,
144]. Thus, it is noticeable that this trend is independent of the raw material. However, the carbon production yield depends on the raw material, as seen in
Table 4.
Table 4. Enhanced carbon production yield by activation with H3PO4.
According to the activated biochar definition [
1,
5,
6,
7,
10], only rice straw, jackfruit peel waste, and rubber wood sawdust are considered activated biochar. The activation mechanism is related to the H
3PO
4:biomass ratio, temperature, and time [
140,
143,
145,
146]. Textural and morphology features are affected depending on time contact and temperature. Low activation time and temperature result in incomplete carbonization and a higher yield [
146,
147]. An appropriate H
3PO
4:biomass ratio, temperature, and activation time lead to improvement of the surface area and pore volume. However, beyond that, those properties can decrease, and it is because the increase in pore size leads to the collapse of the tiny pores [
146].
H
2SO
4 and HNO
3 have also been employed as activating agents of biochar. In general, the presence of H
2SO
4 during the biochar synthesis does not alter its structural properties (e.g., specific surface area and pore volume). However, this acid promotes the sulfonation reaction, generating polar functional groups (e.g., sulphonic groups –SO
3H) at its surface [
119,
148,
149], which, in turn, enhances its performance for several applications like ion and pollutant adsorption [
148,
150,
151], biodiesel production [
152,
153] and other catalytic processes [
119,
154]. Likewise, HNO
3-based species can modify the physicochemical properties of biochar-based materials and, thus, their performance in a specific application. Its presence promotes the generation of many types of surface functional groups through the oxidation and nitration of aromatic rings on the surface of biochar-based materials [
132].
Moreover, HNO
3 can remove partially combusted volatiles and impurities from the surface of biochar, enhancing its surface area and pore volume [
155]. Güzel et al. [
156] and Hadjittofi et al. [
157] have demonstrated that nitric acid-activated biochar-adsorbents can effectively remove methylene blue and Cu
2+ from aqueous solutions, respectively. In both cases, the activated carbonaceous materials exhibited higher adsorption capacities than non-activated biochar, attributed to the larger surface area, the lower point of zero charges, and more oxygen functional groups, like carboxylic, phenolic, and lactonic moieties.
On the other hand, using bases during the biochar activation can generate positive electrostatic charges on their surface, which generates a solid affinity for adsorbed negatively charged pollutants [
119]. Among the bases used as biochar activators, KOH has been widely used because of the special features that it gives to biochar. Biochar properties (e.g., textural and morphological) can be improved using this chemical. The activation properties depend on the KOH: biochar ratio, temperature, and time [
158,
159,
160]. Porosity development is associated with gasification (CO
2 production) [
161]. Different authors report different values of reached specific surface areas: 621 m
2/g [
161], 912.73 m
2/g [
159], and 2201 m
2/g [
160]. Their results differ due to the previously mentioned parameters and the synthesis process. Higher surfaces are obtained when the first raw material is converted to biochar followed by a post-chemical activation (KOH) [
158,
160] rather than direct one-pot pyrolysis and chemical activation [
159,
161]. Likewise, Trakal et al. [
162] studied the effect of chemical activation on the removal efficiency of Cu from an aqueous solution using pure amorphous biochar and activated biochar (BC
act). In this work, chemical activation with 2 M KOH substantially raised the total pore volume of biochar, obtaining values of 0.01 and 8.74 mL/g for amorphous biochar (surface area = 9.80 m
2/g) and BC
act (surface area = 11.6 m
2/g), respectively. These results correlated with the Cu adsorption capacity, which was more significant for BC
act (10.3 mg/g) than that obtained with amorphous biochar (8.77 mg/g).
1.5. Properties That Biochar Modification Processes Can Improve
Biochar modifications can enhance its structure and physicochemical properties (e.g., an increase in the surface area, the generation of oxygen-containing functional groups, and the increase in aromaticity, among others [
163]), favoring its ability to adsorb contaminants, such as heavy metals [
164]. It is due to generating active sites for specific uses, like in catalysis, water treatment, anaerobic digestion, soil remediation [
93], supercapacitors, and fuel cell applications [
118].
The pore size and surface functional groups of biochar are significant features that influence its efficiency as a pollutant adsorbent [
165,
166]. The surface functional groups in biochar are responsible for their strong metal adsorption ability [
164]. Metal adsorption by biosorbents can occur via complexation between metals and different functional groups on the biosorbent surface or through electrostatic attractions between metal cations with negative charges and the functional groups at its surface [
163]. According to Choudhary et al. [
167], functional groups can act as adsorption sites for metal attraction and are located throughout the biochar matrix. In this sense, it is necessary to smash the biochar structure to expose a higher amount of functional groups and, therefore, to promote its efficiency for pollutant removal [
167]. Considering this fact, heavy metal adsorption by biochar can occur at its surface (outer pores) as well as within the pore structure of the carbonaceous material (inner pores), depending on the type and amount of surface functional groups [
164]. Likewise, the removal of other types of pollutants, like dyes [
140,
168,
169], oil [
170], pesticides [
171], and pharmaceuticals [
93,
172], using biochar can occur through monolayer adsorption. During these treatment processes, the chemisorption predominates through the complexation, coordination, ion exchange, and chelation between pollutants and the carbonaceous materials surface, depending on the functional groups and the structural and other physicochemical properties of the biosorbents. These biochar properties depend on the raw material, synthesis method, activation routes, and the use of dopants, composites, and additives described below.
1.6. Dopants, Composites, and Additives Used to Improve Biochar Properties
Many attempts have been made to activate biochar without external doping agents, such as gas, steam, microwaves, acids, alkalis, and oxidants [
118,
129]. On the other hand, adding other materials to the biochar structure has been a novel strategy to produce composites with interesting properties, which can be used in several applications. Some of these strategies and applications of the carbonaceous materials are described in
Table 5:
Table 5. Some chemicals used to modify the properties and applications of biochar samples prepared by pyrolysis.
1.6.1. Dopants for Biochar
Adding a precursor or dopants can improve the physicochemical properties of biochar-based materials. Dopants promote the carbonaceous material’s reactivity, making it an interesting material for catalytic applications. Metallic and non-metallic dopants have been widely used. For example, the modification of biochar with transition metals, like iron, can enhance its specific surface area and the adsorption affinity. In contrast, modifications with non-metals and alkali/alkaline earth metals can decrease the property above [
173]. Mašek et al. [
44] have reported that potassium doping can increase the carbon-sequestration potential of biochar by 45%, making it an important strategy to prevent global warming.
When dopants modify biochar, its functionality can be altered and could improve its performance for several environmental and energetic applications. Minerals, inorganic species, metals, and metal oxides have been the most common dopants to functionalize the biochar structure. Their presence in the carbonaceous matrix displays a significant improvement in adsorption performance, as well as in the selectivity of certain pollutants [
174]. Jha [
175] studied the effect of three chemical dopants on pollutant absorption using biochar-based adsorbents. These dopants were zinc oxide (ZnO), thiol (–SH), and manganese oxide (MnO
2), which exhibited the highest pollutant removal. Other types of dopants have been used to promote the physicochemical properties of biochar and, therefore, its performance in a specific application.
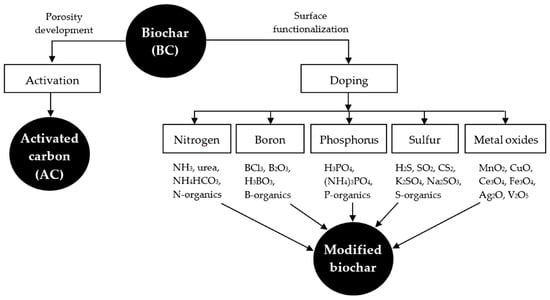
Figure 4 shows some dopants and precursors used to enhance the biochar surface.
Figure 4. Elements and compounds used to improve the biochar structure. (Reprinted from [
120]). © 2018 by the authors.
Doping techniques and procedures, such as impregnation, are the most common methods used for generating changes in the structure of biochar. Di Stasi et al. [
176] produced activated biochar by wet impregnation using cerium nitrate hexahydrate or urea as dopant agents. The aqueous solutions were stirred at 80 °C until the water evaporated entirely. Subsequently, the samples were dried at 110 °C and then calcined in a reactor at 550 °C for 3 h in an inert environment (N
2 atmosphere).
For water purification, well-developed porosity and hydrophobic surfaces are required to effectively enhance the adsorption capacity of organic or inorganic pollutants on biochar-based sorbents. The adsorption of inorganic or polar organic contaminants requires the presence of surface oxygen functional groups to improve the electrostatic attraction [
60]. Unfortunately, sometimes biochar has a moderate to low surface area and a limited number of surface functional groups, which limits their performance [
129]. For this reason, it is necessary to functionalize the biochar surface to improve its properties and, thus, its performance in a specific application. The surface chemistry of biochar can also be altered by doping heteroatoms such as N, P, S, and metal oxide from various sources [
120]. Some modifications have been proposed to improve the adsorption capability of biochar-based materials, which are described below.
1.6.2. Iron-Doped Biochar
Among the dopants used during biochar synthesis, one of the most common and effective has been iron and its species, like iron oxide (Fe
2O
3) [
177,
178,
179].
The presence of iron species in biochar can promote several properties and enhance its effectiveness in various applications. Iron species on the biochar are crucial in immobilization mechanisms and redox reactions [
180,
181,
182]. They can enhance the biochar’s ability to retain essential plant nutrients, such as nitrogen and phosphorus, by forming complexes with nutrient ions, such as nitrates and phosphates [
183]. In addition, iron can help buffer the pH of soils. It acts as a pH stabilizer, preventing extreme fluctuations in soil acidity or alkalinity [
184]. Iron can reduce or oxidize various metals and organics. In the presence of iron, contaminants like arsenic or nitrate can undergo redox reactions that enhance their removal.
Table 6 presents diverse Fe-doped biochar samples from various feedstocks, detailing synthesis conditions and contaminant removal efficiencies. Remarkable examples include peanut hulls, which achieve 98% removal of Cr6+ through hydrothermal carbonization (HTC), and oak wood/bark biochar, which exhibits high removal rates (>98%) for Pb and Cd via pyrolysis and impregnation processes.
Table 6. Fe-doped biochar samples and their synthesis conditions and removal efficiencies.
1.6.3. Nitrogen-Doped Biochar
Nitrogen (N) doping has attracted much attention as it can enhance the characteristics of carbon-based materials [
192]. Because of the significant electronegativity of N, electron modulation can improve the surface polarity of biochar and generate unique electronegativities [
193]. Moreover, N-doping into the biochar matrix can alter its electronic structure, enhancing its interaction with pollutants [
194]. In addition, introducing N heteroatoms into the ordered sp
2-hybridized graphite structure can modify the electrical charges of the original electron network due to the difference in electronegativity. Thus, unbalanced charged areas throughout the carbon structure can result in an electroactive state that may be used for various practical purposes. Likewise, it has been found that N-doping can improve the catalytic activity of nanocarbons, favor nanomaterial dispersion, and increase the detection limit of sensors [
195].
The most common synthesis method for N-doped carbonaceous materials is the thermal decomposition of an inherent N-rich precursor [
192]. It involves chemically pre- or post-treating biochar with ammonia, urea, melamine, or an N-containing organic polymer to add exogenous nitrogen into the carbon structure [
196].
1.6.4. Phosphorus-Doped Biochar
Another way to enhance the biochar properties and performance in a specific application is by doping phosphorus species into the biochar structure. Including these phosphorous-based dopants aims to improve the pollutant removal capacity of biochar. Phosphoric acid (H
3PO
4) is a typical dopant that enhances biochar properties. Fan et al. [
197], have prepared a series of novel N- and phosphorus-enriched biochar nanocomposites via co-pyrolysis with different ammonium polyphosphate (APP) weight ratios. They used the mixture of phosphorus and N dopants to improve the Pb
2+ adsorption on the APP-doped biochar, observing that the Pb
2+ removal efficiency of this last sorbent (723.6 mg/g) was significantly enhanced compared to that of the unmodified biochar (264.2 mg/g).
1.6.5. Composites for Biochar
Adding a composite material to the biochar structure can be an interesting enhancement strategy to improve its environmental remediation efficiency. Metal composites (e.g., Fe
2O
3 and iron sulfide), minerals (e.g., kaolinite), and layered double hydroxides (LDH) have been the typical composites used to promote the performance of the carbonaceous material during soil remediation (resulting in fertility improvements) and wastewater treatments [
198]. LDHs are anionic clay minerals made up of positively charged metal hydroxide layers and anions in the interlayer gap to neutralize charge [
199]. In pollutant adsorption, various LDH-biochar composites with divalent and trivalent metal cations (e.g., Mg-Al, Mg-Fe, Zn-Al, Ca-Al, and Ni-Fe) have been frequently used [
200].
1.6.6. Other Additives for Biochar Modification
Other additives, such as phosphorus, zinc, and calcium species, can be added to biochar during its synthesis to improve its properties and broaden its applicability [
201]. For example, adding calcium oxide to biochar followed by a heating process at 450 °C can generate a more stable carbonaceous material with fewer oxygen functional groups [
202]. According to Li et al. [
203], adding mineral additives to biochar promotes carbon retention and the stability of the solid in terms of carbon sequestration. They studied the use of kaolin, calcite (CaCO
3), and calcium dihydrogen phosphate [Ca-(H
2PO
4)
2] as additives in biochar obtained from rice straw biomass. These three minerals are frequently used to enhance soil quality and remediate soil and water pollution [
204]. Likewise, adding these chemicals to biochar can improve the stability of the biochar-based material and, thus, its efficiency in removing pollutants [
8].
2. Main Uses of Biochar
2.1. Biochar for Soil Remediation (Crop Improvement)
The presence of biochar can enhance soil characteristics and, at the same time, increase crop biomass and improve disease resistance. Biochar may improve soil fertility [
205], soil quality (e.g., pH [
40], cation exchange capacity (CEC), and water holding capacity [
206]), and plant development [
207,
208]. Recently, biochar has been used to treat soil contaminated with heavy metals and organic contaminants [
43].
2.2. Biochar to Remove Pollutants in Water and Wastewater
Sources of water pollution can be classified in different ways. The most important sectors that generate wastewater or contribute to its pollution are domestic, agricultural, and industrial [
209,
210]. The word “contaminant,” according to the Safe Drinking Water Act, is defined as any physical (sediment or organic material suspended in the water), chemical (nitrogen, bleach, salts, pesticides, metals), biological (bacteria, viruses, protozoa, and parasites), or radiological (cesium, plutonium, and uranium) substance or species in water [
211]. Some pollutants in drinking water may be dangerous or toxic at specific concentrations in drinking water, while others can be innocuous. The presence of pollutants does not always imply that the water is unsafe to drink. However, it is necessary to consider how to remove them.
Table 7 shows the sub-classifications and various sources of water pollution.
Table 7. Sources of wastewater generation [
143].
Biochar’s physical and chemical properties are influenced by the feedstock, synthesis method, and activation procedures, and its adsorption capacities are also influenced. This carbonaceous material can be used to remove a wide range of organic (agricultural), inorganic (toxic gases in the oil industry), and microbiological (pathogen) pollutants [
213].
Table 8 describes some examples of types of contaminants in water that can be removed using biochar-based materials.
Table 8. Frequent pollutants of water generated in industrial areas [
145].
| Type of Pollutant |
| Organic |
Inorganic |
Microbial |
| Dye, humid substances |
Heavy metals |
Bacteria |
| Phenolic compounds |
Inorganic ions |
Mushrooms |
| Petroleum surfactants |
Pb2+ |
Salmonella |
| Pesticides, pharmaceuticals |
Zn2+ |
Enterococcus faecalis |
| Compounds |
Cd2+ |
In addition to classifying the origin of wastewater, it is necessary to subdivide the types of contaminating agents in water and their effects on the hydric fluid. Table 9 describes the main contaminating agents, such as organic and inorganic agents, and their environmental effects.
Table 9. Polluted water and its environmental effects [
146,
147,
148,
149].
Biochar treatment has a high potential for wastewater treatment [
47,
214]. Compared to conventional low-cost technologies (such as sand filtration, boiling, sun disinfection, and chlorination), water treatment using biochar has numerous potential advantages: (1) biochar is a low-cost and renewable adsorbent made from readily available biomaterials and skills, making it suitable for low-income communities; (2) existing methods primarily remove pathogens, whereas biochar removes chemical, biological, and physical contaminants; and (3) biochar preserves the organoleptic properties of water, whereas existing methods generate carcinogenic by-products (e.g., chlorination) and increase chemical contaminant concentrations (e.g., boiling) [
213].
Biochar has been widely explored as an adsorbent for removing contaminants from wastewater due to its unique features, such as a large surface area, well-distributed pores, and a high abundance of surface functional groups [
215]. The oxygenated functional groups (OFGs) in biochar are essential active sites for removing contaminants from the water via interfacial adsorption/redox reaction [
214,
216].
2.2.1. Mechanisms to Remove Pollutants from Water with Carbonaceous Materials
Low-cost biochar has emerged as the substitute for activated carbon for the removal of organic pollutants such as volatile organic compounds, aromatic dyes, hydrocarbons, agrochemicals, and others. Regarding inorganic contaminants, biochar has been successfully used for the removal of sulfides, ammonia, nitrates, phosphate, and heavy metals [
92]. The application of biochar as an efficient contaminant remover depends on its remarkable characteristics, e.g., high specific surface area, cation exchange capacity, active functional groups, microporosity, and electrostatic interactions, among others. These properties govern the binding of polar compounds on the surface of biochar, which immobilizes the contaminants. Because of all this, biochar has been proposed in many reports as an efficient adsorbent to remove different types of organic and inorganic contaminants from water and soil in the near future. The adsorption of inorganic pollutants on biochar results from stoichiometric ionic exchange, electrostatic attraction, surface precipitation, surface sorption, and complexation [
217]. In this sense, the adsorptive capabilities of biochar are influenced by various factors, including hydrophobicity, alkalinity, ion exchange capacity, and elemental compositions [
218]. Surface functionality can also alter the biochar sorption capacity [
219]. Rajapaksha et al. [
220] have reported a mechanism of contaminant removal in water through the strong interaction between organic compounds and carbon membranes. A recent report [
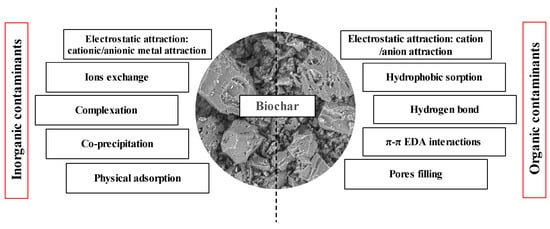
92] has summarized these processes to remove inorganic contaminants as a combined effect of several types of interactions, such as electrostatic interactions due to a high dependency on the point of zero charge, surface sorption because of the diffusion of the metal ions onto the pores of the sorbent, and chemical bonds with active functional groups. Also, via cation exchange as a result of the replacement of positive charges on the surface of biochar by metal ions, complexation takes place because of the oxygen functional group (for example, carboxyl and phenolic) with high efficiency of binding heavy metal ions. On the other hand, the removal of organic contaminants can also be connected with the combination of different interactions. These interactions are mainly hydrophobic interactions, pore filling, partitioning, electron donor and acceptor, and electrostatic interactions. The contaminants can be attracted to the carbonaceous membranes (e.g., graphene and biochar) through intermolecular forces, such as non-covalent bonds, hydrogen bonds, van der Waals forces, π–π stacking, and hydrophobic interactions. The mechanism of removal of contaminants by carbonaceous materials is illustrated in
Figure 6.
Figure 6. Mechanism of pollutant removal from water using granular carbonaceous materials (Reprinted from [
221]). © 2020 by the authors.
2.2.2. Biochar Used at Medium and Large-Scale in Water Filtering Process
The global demand for safe and quality drinking water has become increasingly important due to the growing world population and anthropogenic activities. Water pollution by synthetic organic compounds, such as pesticides, medicines, and fuel components, is an increasing concern worldwide because these chemicals can bioaccumulate in the human body, causing cancer and other disorders. In recent years, many researchers have focused on the applications of biochar as a potential and efficient adsorbent to remove contaminants from aqueous solution. Due to its remarkable properties, numerous reports have been published confirming the many advantages of biochar for environmental uses, and it has been widely studied in removing both organic and inorganic contaminants [
64]. As an efficient adsorbent, it has been used to immobilize heavy metal ions, even as a catalyst for the degradation of complex organic compounds. Nevertheless, the industrial application of these carbonaceous materials requires significant infrastructure expenditures [
222,
223]. Considering this fact, creating filters with carbonaceous materials at different scales becomes an excellent option to mitigate or reduce aquatic pollution at different scales. The use of biochar filters has been suggested as an option to replace both treatments of drinking water: the conventional treatment (e.g., coagulation-flocculation, filtration, and chlorination) and the advanced treatment (e.g., membrane filtration, ozonation, and biofiltration) [
3].
Some authors have also compared the advantages of using biochar for water treatment to low-cost methods [
213]. They consider that biochar treatment has several merits compared to methods such as sand filtration, boiling, solar disinfection, or chlorination because, although some methods remove pathogens, biochar removes chemical, biological, and physical contaminants. Moreover, it maintains the organoleptic properties of water, while other treatments, such as chlorination, might produce carcinogenic by-products [
213]. Recent work has focused on using engineering biologically enhanced biochar (BEB) for biological water treatment [
210], focusing on the scope, potential benefits, and challenges of sustainable water treatment using BEB. The work examines BEB’s dynamic and complex biofilm–biochar interactions in water treatment. The authors also suggest the use of BEB instead of biological activated carbon (BAC) in the tertiary treatment of drinking water due to the immobilization of microbes on the surface facilitating contaminants removal via a combined adsorption and biodegradation process, on the basis that the biofilms can degrade and remove a wide range of organic, inorganic, and biological waterborne contaminants.
Inexpensive and available biochar and woodchips were used for anaerobic wastewater filtration, and their suitability was evaluated compared to gravel as a standard reference material [
224]. Filters were fed with raw sewage from a municipal full-scale wastewater treatment plant in Germany at room temperature. The performance of the biochar filters was much better over the experiment compared to woodchip and gravel filters concerning chemical oxygen demand, total organic carbon, turbidity, and fecal indicator bacteria removal efficiency, showing the superior properties of biochar for wastewater treatment. Advanced oxidation processes are proven to be efficient in water treatment (reduction of toxic, organic pollutants) and elimination of emerging concerns like pollutants (toxins, pesticides, dyes, etc.) and include UV/O
3, UV/H
2O
2, Fenton, photo-Fenton, nonthermal plasmas, sonolysis, photocatalysis, radiolysis, supercritical water oxidation processes, etc. [
225]. In this point, it is very important to mention that advanced oxidation can be achieved using biochar because of the radical groups, mainly hydroxyl radical, introduced by chemical treatments such as acid or alkali hydrolysis. Biochar functionalized with hydroxyl groups enhances soil structure and reduces soil erosion, facilitates water and nutrient retention, etc.
This entry is adapted from the peer-reviewed paper 10.3390/resources13010008