Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Radiologists and oncogynecologists should be familiar with different liver shape variations to avoid diagnostic errors and unwanted intraoperative surgical complications. Surgeons should be aware of variations in liver shape as in such cases, the vasculature or gallbladder may have a variant anatomical location.
- liver morphology
- liver anatomy
- ovarian cancer surgery
1. Liver Shape Variations
The normal liver is wedge-shaped, with the narrow end pointing to the left. It resembles a five-sided pyramid [1][2][3][4]. However, the liver can have globular, quadrilateral, rectangular, square, conical, boot, or saddle shapes [1][2][3][4]. Srimani and Saha investigated 110 isolated formalin-fixed livers from adult cadavers. The authors found a normal wedge liver shape in 57.3% of the specimens. The other 42.7% of the livers had shape variations, of which quadrilateral (14.5%) and transverse saddle shapes (9.1%) were more common [3].
Significance of Liver Shape Variations in Ovarian Cancer Surgery
Radiologists and oncogynecologists should be familiar with different liver shape variations to avoid diagnostic errors and unwanted intraoperative surgical complications. Surgeons should be aware of variations in liver shape as in such cases, the vasculature or gallbladder may have a variant anatomical location (Figure 1).
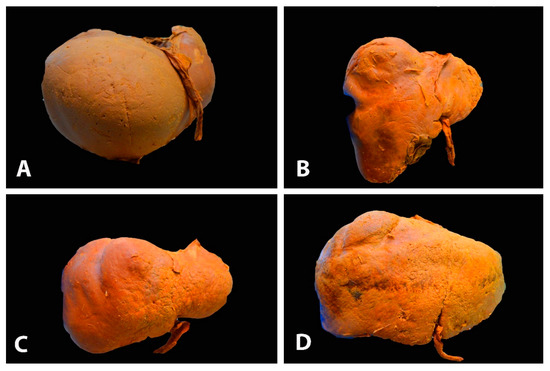
Figure 1. Different gross shapes of the liver—anterior surface of the liver (author’s own material): (A) globular shape; (B) conical shape of the right liver lobe and the small left lobe; (C) quadrilateral shape; (D) rectangle shape.
2. Congenital and Acquired Variations of the Liver
Congenital liver variations commonly include accessory lobes, agenesis, or atrophy of lobes. In contrast, acquired variations develop over the course of an individual’s lifetime. They result from diaphragmatic, peritoneal, or other organ pressures over the liver [2]. The most widely used classification of morphological variations of the liver is the Netter’s classification (Figure 2) [5][6].
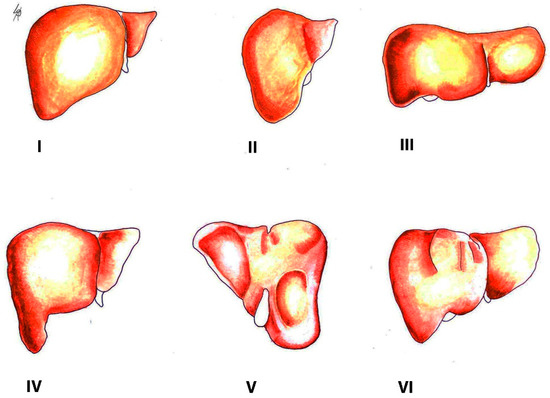
Figure 2. Morphological variations of the liver according to Netter’s classification (modified from references [5][6]). Type I—small left liver lobe and deep costal impression; Type II—left lobe atrophy; Type III—saddle-like liver with hypertrophied left lobe; Type IV—Riedel’s lobe; Type V—deep renal impression and corset construction; Type VI—diaphragmatic grooves.
There are few studies on morphological variations of the liver as anatomists and hepatobiliary surgeons are more interested in the branching pattern of the hepatobiliary system and the vasculature of the liver [1][2][3][4][5][6][7]. Gross variations of the liver include diaphragmatic grooves, accessory fissures, agenesis or accessory lobes, and pons hepatis [1][2][3][4][5][6][7]. The most important gross variations of the liver associated with ovarian cancer surgery are diaphragmatic grooves, accessory fissures, Riedel’s lobe, hypertrophied papillary process (Spiegel’s lobe) or caudate process, and pons hepatis.
2.1. Accessory Liver Fissures
The presence of accessory liver fissures in different lobes of the liver is the most common morphological variation. It should be stated that such fissures could be observed in all lobes of the liver. However, they are commonly found on the visceral surface of the liver. Additionally, they can be single or multiple [1][2][3][4][7]. Singh and Rabi [2] observed 70 formalin-fixed livers and found the presence of accessory fissures in 81.4% of all examined livers. Vinnakota and Jayasree investigated 58 livers and found an incidence of accessory liver fissures in 53.44% [4].
2.2. Deep Diaphragmatic Grooves
Prominent vertical diaphragmatic grooves are most often found on the anterosuperior surface of the right lobe of the liver and rarely on the left. They can be single or multiple, ranging from two to six. Their depth ranges from 1 cm to 2 cm and the corresponding serosa is intact [1][2][3][8]. There is a difference between the sexes as these grooves are observed more frequently in women than men [8]. The presence of diaphragmatic grooves varies from 6% to 11.43% [1][2][3]. However, Macchi et al. [8] examined 48 human livers and found an incidence of these grooves of 40%. The deep diaphragmatic grooves are considered to be acquired as they are believed to result from costal pressure and invagination of the diaphragm muscle into the liver [1][2][3]. Mancchi et al. [8], however, concluded that these grooves could be a suitable marker for the portal fissures and for the superficial projection of the hepatic veins with their tributaries.
Deep diaphragmatic grooves and liver fissures are shown in Figure 3.
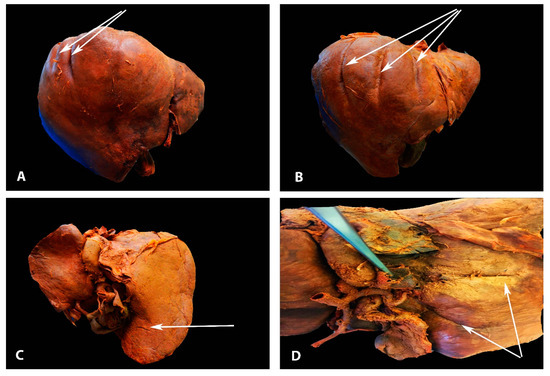
Figure 3. Deep diaphragmatic liver grooves and liver fissures (author’s own material). (A)The arrows show two diaphragmatic grooves on the right liver lobe (anterior liver surface). (B) The arrows show three diaphragmatic grooves on the right liver lobe (anterior liver surface). (C) The arrow shows liver fissure on the right lobe (posterior liver surface). (D) The arrows show two liver fissures on the right lobe (posterior liver surface).
2.3. Significance of Liver Fissures and Grooves in Ovarian Cancer Surgery
Liver fissures and grooves are of great clinical significance in ovarian cancer surgery as they represent potential sources of diagnostic imaging errors (particularly computed tomography (CT) imaging). Any fluid source in the grooves can mimic a cyst or metastatic tumor in the liver, an intrahepatic hematoma, or a liver abscess. Moreover, disseminated ovarian cancer cells in the diaphragmatic grooves or hepatic fissures could be mistaken for intrahepatic focal lesions [9]. Therefore, a thorough knowledge of the anatomy and variations of the liver surface may help to avoid unnecessary misdiagnosis.
Diaphragmatic grooves could be mistaken with Chilaiditi sign or syndrome, especially in cases of free air due to a perforation of abdominal organ [10].
Chilaiditi sign (CS) is defined as interposition of the colon (commonly transverse mesocolon) between the right liver lobe and the diaphragm. The sign represents a radiological finding of a gas between the right diaphragm and the right lobe of the liver [11][12][13][14][15].
The CS is known as Chilaiditi syndrome when it is accompanied by symptoms (pain, vomiting, constipation) and complications (intestinal obstruction, perforation, and ischemia) [10][11][12][13][14][15]. There are many theories for this untypical predisposition of the bowel—diaphragmatic (phrenic nerve palsy or congenital muscle loss), hepatic (weakness of the falciform ligament), abnormally long colon, ascites, and obesity [10][13][14][15]. There is also a theory which states that the diaphragmatic grooves are formed by a mesocolic tissue invasion of the adjacent anterior right liver lobe margins [11]. Yavuz et al. noticed the possible relation between diaphragmatic grooves, CS, and Chilaiditi syndrome. Therefore, the authors retrospectively investigated this possible connection on 2314 CT scans. The authors did not find statistical or significant correlation between diaphragmatic grooves and the syndrome. However, the authors concluded that the grooves are likely derived from the CS, as more than half of the patients with CS had diaphragmatic grooves (25 patients of 46 (54.3%) had grooves on the right liver lobe near the falciform ligament). Their theory is supported by the fact that the majority of grooves are found among the adult population. Nevertheless, Yavuz et al. mentioned that further studies are needed [11].
It should be stressed that Chilaiditi syndrome could be confused radiologically with diaphragmatic grooves. Cawich et al. reported a case of a patient with peptic ulcer perforation at the first part of the duodenum. The authors initially considered possible Chilaiditi syndrome as the patient had an air above the right lobe of the liver on preoperative radiographs. Intraoperative findings showed diaphragmatic grooves on the right liver lobe. Authors concluded that a true pneumoperitoneum with the presence of diaphragmatic grooves could be mistaken with Chilaiditi syndrome [10].
3. Riedel’s Lobe
Riedel’s lobe is defined as a downward tongue-like projection of the anterior edge of the right liver lobe. Riedel’s lobe is located right to the gallbladder. In the medical literature, it is also termed a floating lobe or “tongue-like” lobe. The incidence varies from 3.3% to 31% [16][17][18][19]. This anatomical variation is rather asymptomatic, although symptoms such as abdominal distension and torsion episodes are possible observations [16]. Riedel’s lobe is shown in Figure 4 and Figure 5.
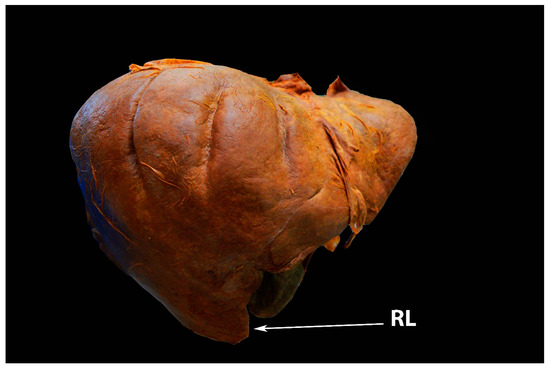
Figure 4. Riedel’s lobe of the liver—anterior liver surface (author’s own material). RL—Riedel’s lobe.
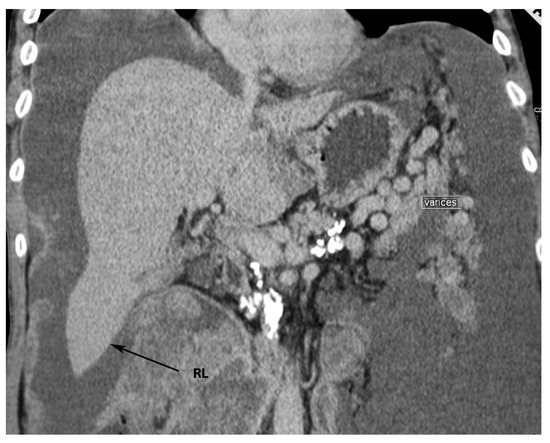
Figure 5. Riedel’s lobe on CT (author’s own material). RL—Riedel’s lobe.
Significance of Riedel’s Lobe in Ovarian Cancer Surgery
Riedel’s lobe can be confused with an enlarged lymph node or an unidentified abdominal mass on various imaging techniques. In cases of isolated metastatic lesions in Riedel’s lobe, resection is a possible option [7][16].
A few cases of primary malignant tumors or metastases to Riedel’s lobe have been described [20][21][22]: Soo et al. reported on a Riedel’s lobe metastasis from a ductal breast cancer [20]; Zamfir et al. observed a case of a 65-year-old woman with primary hepatocellular carcinoma arising from her Riedel’s lobe. The lobe was resected with “en-block” cholecystectomy [21]. Al-Handola et al. reported a case of 64-year-old woman with incidental observation of Riedel’s lobe and intrahepatic cholangiocarcinoma. The authors stated that there are unanswered associations between Riedel’s lobe and cancer. They concluded that the lobe could be considered a possible site for primary hepatocellular carcinoma or hidden metastases [22]. Notably, the majority of cases of Riedel’s lobe involvement by a malignant tumor affected the female population [20][21][22]. However, there is no reported case in medical literature of metastases to Riedel’s lobe by ovarian cancer. Perhaps there were such cases, but this liver pathology was probably neglected by oncogynecologists. Moreover, some authors believe that the lobe is a simple variant of liver anatomy, corresponding to hypertrophy of segments V and VI, rather than a true anatomical variation [23][24][25]. Additionally, the lobe can be a source of a living-related hepatic transplant [7][16]. Therefore, ovarian cancer metastases to Riedel’s lobe should be staged as FIGO stage IV, as this liver anomaly is actually part of the liver.
4. Papillary Process (Spiegel’s Lobe) and Caudate Process of the Caudate Lobe
The caudate lobe of the liver consists of three parts—the caudate process (lateral), the paracaval part, and the medial papillary process (medial). The papillary process is also known as Spiegel’s lobe. The medial papillary process can be hypertrophied, prominent, underdeveloped, and absent [1][2][3][7][26][27]. Singh and Rabi observed an enlarged and underdeveloped papillary process in 4.29% and 1.43% of 70 examined liver specimens [2]. Srimani and Saha found an enlarged papillary process in 21.8% of 110 livers examined [3]. The authors also observed the absence of the caudate and papillary processes in 16.4% of the investigated specimens [3]. Another study found a prominent papillary process in 32% of 90 formalin-fixed livers [28].
Significance of the Papillary Process (Spiegel’s Lobe) and Caudate Process of the Caudate Lobe of the Liver in Ovarian-Cancer Surgery
A normal or small papillary process of the caudate lobe can be mistaken for enlarged lymph nodes at porta hepatis on CT scans. In contrast, an elongated Spiegel’s lobe or caudate process might mimic a pancreatic body mass [27].
The hypertrophied Spiegel’s lobe of the liver is shown in Figure 6. The hypertrophied caudate lobe of the liver is shown in Figure 7. The elongated papillary process is shown in Figure 8.
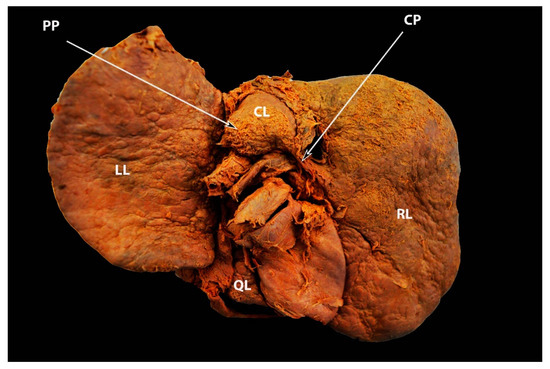
Figure 6. Elongated caudate process and hypertrophied papillary process showed by arrows—posterior liver surface (own material). LL—left lobe; RL—right lobe; QL—quadrate lobe; CL— caudate lobe; CP—caudate process; PP—papillary process.
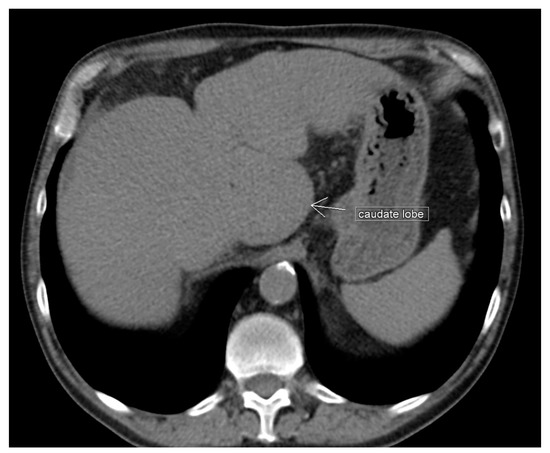
Figure 7. Unenhanced transverse abdominal CT liver level with isolated caudate lobe hypertrophy (own material). The arrow shows isolated caudate lobe hypertrophy.
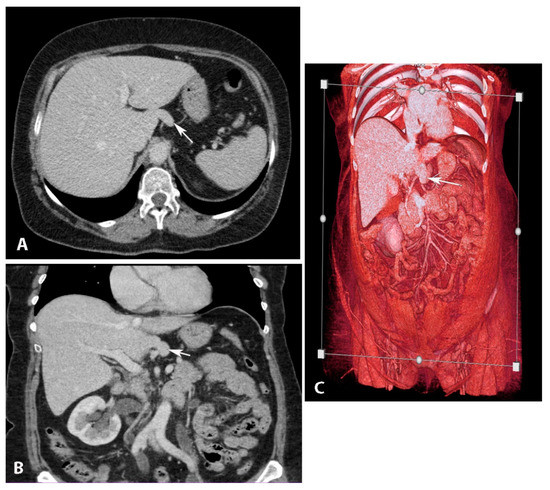
Figure 8. Elongated papillary process (author’s own material). (A) Transverse abdominal CT at the level of the liver with elongated papillary process present. (B) Coronal abdominal CT where papillary liver process projection can be seen just above the portal vein. (C) CT Volume rendered image of the abdomen showing the papillary liver process in its typical location. Arrows show elongated papillary process.
5. Pons Hepatis
Pons hepatis, also known as pont hepatique, is defined as bridging over the ligamentum teres fissure between the quadrate and left lobes of the liver. The anatomical variations can be divided into three subtypes: I—no communication; II—membranous communication; III—a large parenchymal bridge, in which the left and quadrate lobes emerge as a connected lobe covering the umbilical vein [29][30][31]. The incidence of pons hepatis varies in the medical literature. Studies examining the morphological anatomy and variations of the liver reported different incidences of 22.86% [2], 30% [28], 35.5% [3]. Pons hepatis is shown in Figure 9.
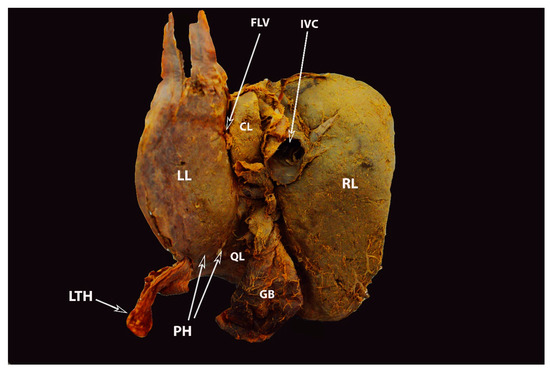
Figure 9. Complete pons hepatis—type III (posterior liver surface). The fissure for ligamentum teres is absent (own material). LL—left liver lobe; RL—right liver lobe; FLV—fissure for ligamentum venosum; IVC—inferior vena cava; QL—quadrate lobe; CL—caudate lobe; GB—gall bladder; LTH—ligamentum teres hepatis; PH—pons hepatis.
Significance of Pons Hepatis in Ovarian Cancer Surgery
The pons hepatis is functionally insignificant but may appear as an extrahepatic mass (on CT scan or ultrasound) if metastatic ovarian cancer spreads here [3][32]. Moreover, in the case of pons hepatis, it may not be possible to observe the fissure of the ligamentum teres on a CT scan and the dimensions of the two main lobes of the liver (left and right) may be incorrect [28]. The pons hepatis, especially the zone where the ligamentum teres hepatis attaches to the liver, is a site of metastatic disease that can carry tumor implants.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13142371
References
- Chaudhari, H.J.; Ravat, M.K.; Vaniya, V.H.; Bhedi, A.N. Morphological Study of Human Liver and Its Surgical Importance. J. Clin. Diagn. Res. 2017, 11, AC09–AC12.
- Singh, H.R.; Rabi, S. Study of morphological variations of liver in human. Transl. Res. Anat. 2019, 14, 1–5.
- Srimani, P.; Saha, A. Liver morphology: Anatomical study about the outer aspects. Surg. Radiol. Anat. 2020, 42, 1425–1434.
- Vinnakota, S.; Jayasree, N. A new insight into the morphology of the human liver: A cadaveric study. ISRN Anat. 2013, 2013, 689564.
- Lau, W.Y. Different Approaches to Liver Resection; Springer: Singapore, 2021; pp. 155–169.
- Netter, F.H. Atlas of Human Anatomy, 2nd ed.; 18 Guilford Press: New York, NY, USA, 2000.
- Phad, V.V.; Syed, S.A.; Joshi, R.A. Morphological variations of liver. Int. J. Health Sci. Res. 2014, 4, 119–124.
- Macchi, V.; Feltrin, G.; Parenti, A.; De Caro, R. Diaphragmatic sulci and portal fissures. J. Anat. 2003, 202 Pt 3, 303–308.
- Auh, Y.H.; Lim, J.H.; Kim, K.W.; Lee, D.H.; Lee, M.G.; Cho, K.S. Loculated fluid collections in hepatic fissures and recesses: CT appearance and potential pitfalls. Radiographics 1994, 14, 529–540.
- Cawich, S.O.; Spence, R.; Mohammed, F.; Gardner, M.T.; Sinanan, A.; Naraynsingh, V. The liver and Chilaiditi’s syndrome: Significance of hepatic surface grooves. SAGE Open Med. Case Rep. 2017, 5, 2050313X17744979.
- Yavuz, A.; Batur, A.; Bulut, M.D.; Bora, A.; Göya, C.; Andic, C.; Beyazal, M.; Olmez, S. Anterior hepatic grooves accompanied by Chilaiditi sign: A retrospective radiological analysis of a neglected anatomical fact. Surg. Radiol. Anat. 2015, 37, 483–492.
- Chilaiditi, D. Zur Frage der Hepatoptose und Ptose im allegemeinen im Anschluss an drei Fallevon temporarer, partieller Leberverlagerung. Fortschr. Geb Rontgenstr. Nukl. 1910, 16, 173–208.
- Gulati, M.S.; Wafula, J.; Aggarwal, S. Chilaiditi’s sign possibly associated with malposition of chest tube placement. J. Postgrad. Med. 2008, 54, 138–139.
- Moaven, O.; Hodin, R.A. Chilaiditi syndrome: A rare entity with important differential diagnoses. Gastroenterol. Hepatol. 2012, 8, 276–278.
- Sohal, R.J.; Adams, S.H.; Phogat, V.; Durer, C.; Harish, A. Chilaiditi’s Sign: A Case Report. Cureus 2019, 11, e6230.
- Savopoulos, C.; Kakaletsis, N.; Kaiafa, G.; Iliadis, F.; Kalogera-Fountzila, A.; Hatzitolios, A.I. Riedel’s lobe of the liver: A case report. Medicine 2015, 94, e430.
- Riedel, I. Ueber den zungenfrmigen Fortsatz des rechten Leberlappens und seine pathognostische Bedeutung für die Erkrankung der Gallenblase nebst Bemerkungen über Gallensteinoperationen. Berl. Klin. Wochenschr. 1888, 25, 577–602.
- Sham, R.; Sain, A.; Silver, L. Hypertrophic Riedel’s lobe of the liver. Clin. Nucl. Med. 1978, 3, 79–81.
- Gillard, J.H.; Patel, M.C.; Abrahams, P.H.; Dixon, A.K. Riedel’s lobe of the liver: Fact or fiction? Clin. Anat. 1998, 11, 47–49.
- Soo, M.S.; Adatepe, M.H. Metastatic lesions arising in a Riedel’s lobe. Findings from a sulfur colloid liver-spleen scan. Clin. Nucl. Med. 1990, 15, 814–815.
- Zamfir, R.; Braşoveanu, V.; Boroş, M.; Herlea, V.; Popescu, I. Hepatocellular carcinoma in Riedel’s lobe. Chirurgia 2008, 103, 121–123.
- Al-Handola, R.; Chinnappan, J.; Bakeer, M.; Ayad, S. Incidental Finding of Riedel’s Lobe of the Liver and Intrahepatic Cholangiocarcinoma. Cureus 2023, 15, e40683.
- Kudo, M. Riedel’s lobe of the liver and its clinical implication. Intern Med. 2000, 39, 87–88.
- Lane, D. Masses in the right hypochondrium and Riedel’s lobe. Med. J. Aust. 1966, 1, 896–899.
- De Simoni, O.; Barina, A.; Gruppo, M.; Scapinello, A.; Mourmouras, V.; Pilati, P.; Franzato, B. A Potentially Misleading Hepatocellular Carcinoma. Medicina 2021, 57, 850.
- Gray, H.; Standring, S.; Hrold Ellis, H.; Berkovitz, B. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone Edinburgh: New York, NY, USA, 2005; pp. 2171–2175.
- Auh, Y.H.; Rosen, A.; Rubenstein, W.A.; Engel, I.A.; Whalen, J.P.; Kazam, E. CT of the papillary process of the caudate lobe of the liver. AJR Am. J. Roentgenol. 1984, 142, 535–538.
- Joshi, S.D.; Joshi, S.S.; Athavale, S.A. Some interesting observations on the surface features of the liver and their clinical implications. Singapore Med. J. 2009, 50, 715–719.
- Mittal, A.; Goyal, G.L.; Kamath, V.G. Variations in Hepatic Segmentation on the Surface of Liver—A Cadaveric Study. JK Sci. 2021, 23, 43–46. Available online: https://journal.jkscience.org/index.php/JK-Science/article/view/43 (accessed on 12 January 2023).
- Donmez, B.O.; Sarikcioglu, L.; Gokhan, G.; Elpek, G.O.; Ucar, Y. Pons hepatis: Report of two cases. Acta Gastroenterol. Belg. 2009, 72, 279–280.
- Couinaud, C. (Ed.) Surgical Anatomy of the Liver Revisited; C. Couinaud 15 rue Spontini F 75116 Paris: Paris, France, 1989.
- Onitsuka, A.; Katagiri, Y.; Miyauchi, T.; Shimamoto, T.; Mimoto, H.; Ozeki, Y. Metastatic hepatoma originating from the pons hepatis presenting extrahepatic growth--classification of different patterns covering REX’s recessus. Hepatogastroenterology 2003, 50, 235–237.
This entry is offline, you can click here to edit this entry!
