Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Thiophenes represent a small family of natural metabolites featured by one to five thiophene rings. Numerous plant species belonging to the family Asteraceae commonly produce thiophenes. These metabolites possessed remarkable bioactivities, including antimicrobial, antiviral, anti-inflammatory, larvicidal, antioxidant, insecticidal, cytotoxic, and nematicidal properties.
- thiophenes
- Asteraceae
- biosynthesis
- bioactivities
- health and wellbeing
- life on earth
1. Introduction
Heterocyclic compounds display a remarkable role in the field of bioactive metabolites search. It is noteworthy that >75% of clinically utilized drugs possess heterocyclic moiety in their chemical skeleton [1]. Sulfur belongs to chalcogens that are the 16 group elements of the periodic table. Sulfur is a ubiquitous heteroatom in medicinal chemistry that can bond to various atoms, including nitrogen, oxygen, carbon, halides, and phosphorus. Several sulfur-based functionalities have become privileged pharmacophores in synthesizing new derivatives that contribute to drug discovery [2]. In living organisms, it displays a remarkable characteristic of possessing a variety of redox potentials and redox states, producing many sulfur species that take part in diverse biological processes. Thioethers and thiols can form sulfonium ions by donating electrons to other organic species, revealing their ability to stabilize a negative charge on a neighboring carbon [3]. They can undergo sequential oxidation to sulfoxides and sulfones, which have diverse biological roles. For example, S-adenosylmethionine (SAM—sulfonium compound) mediates most biochemical methylation reactions in cell metabolism [4].
S-containing species have featured a strong electron-withdrawing nature, resistance to reduction at sulfur, stability against hydrolysis, and preference for two electrons over radical processes that make this group of compounds applicable to many drug research fields [5]. Their diverse pharmacological potential makes it the first choice for incorporation by the hybrid approach, which is present in most of the required medicines accessible in the market [5]. It was reported that 41 sulfur-containing commercial drugs appeared in the Top 200 Pharmaceuticals by Retail Sales in 2019 worldwide; 20.5% contain a sulfur atom [6].
Natural products have attracted significant attention as a potential source of S-containing compounds for drug discovery. The well-known conotoxin, ecteinascidin 743 (ET-743), and penicillin are examples of natural sulfur-containing clinical drugs. Furthermore, many sulfur-containing drugs are derived from natural products, e.g., phthalascidin and ixabepilone for cancer treatments, rosuvastatin for hyperlipidemia, and dalfopristin and quinupristin for infectious diseases [7].
Thiophenes are among the heterocyclics that have been located in the focus of research interest for the last decades. They are a class of sulfur-containing molecules usually composed of one to five thiophene units connected at the α-position and often have various alkyl groups at the α’-carbon of the terminal ring [8]. Thiophene derivatives have beneficial applications in the dye, pharmaceutical, and agrochemical industries [9][10]. Interestingly, many of the approved drugs available in the markets have thiophene moiety, including antiasthma, NSAIDs (non-steroidal anti-inflammatory drugs), diuretics, anticancer, and antihistaminic drugs [11][12]. Natural occurring thiophenes represent rare constituents reported from these metabolites that have been isolated from various Asteraceae genera: Echinops, Eclipta, Pluchea, Artemisia, Tagetes, Porophyllum, Atractylodes, Atractylodes, and Xanthium. Additionally, some are reported from Ferula (family Apiaceae), as well as from actinomycetes (Streptomyces) and fungi (Penicillium) (Figure 1) [8].
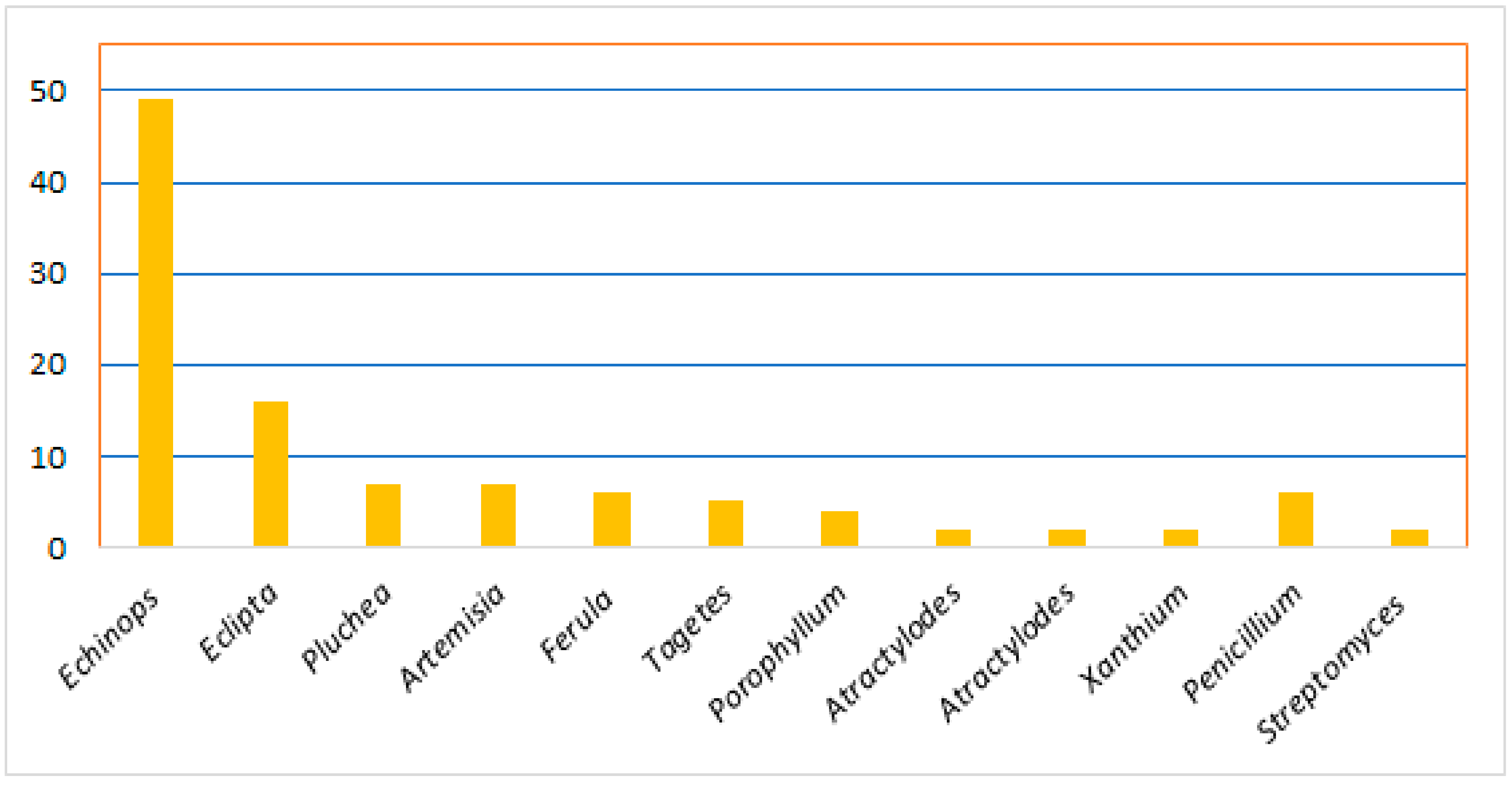
Figure 1. Number of reported thiophenes from various sources.
They are produced as a chemical defense mechanism and are toxic to various pathogens, such as insects, nematodes, bacteria, and fungi [13][14]. Biosynthetically, they are derived from fatty acids or polyacetylenes through acetylene intermediates; therefore, they are named acetylenic thiophenes. Indeed, many of the reported derivatives possess an alkyl chain with an acetylenic unit that may contain chiral centers due to introducing a hydroxy group [8]. These metabolites have remarkable biological and pharmacological effectiveness, including antiviral, antimicrobial, antileishmanial, anti-inflammatory, larvicidal, antioxidant, insecticidal, HIV-1 (human immunodeficiency virus-1) protease inhibitory, cytotoxic, nematicidal, and phototoxic effects [8][15][16][17][18][19][20] (Figure 2).

Figure 2. Biological activities of thiophenes.
In the previous research, 96 natural thiophene derivatives were listed from various plant species belonging to the Asteraceae family till 2015, with a particular focus on their biosynthesis, bioactivities, and physical and spectral data [8]. Recently, several reviews dealing with synthetic thiophene-based derivatives, including their anti-inflammation and anticancer potentials, spectroscopic properties, and synthesis, were published [21][22][23][24]. On the other side, there is no available review on naturally occurring thiophene derivatives from plant sources.
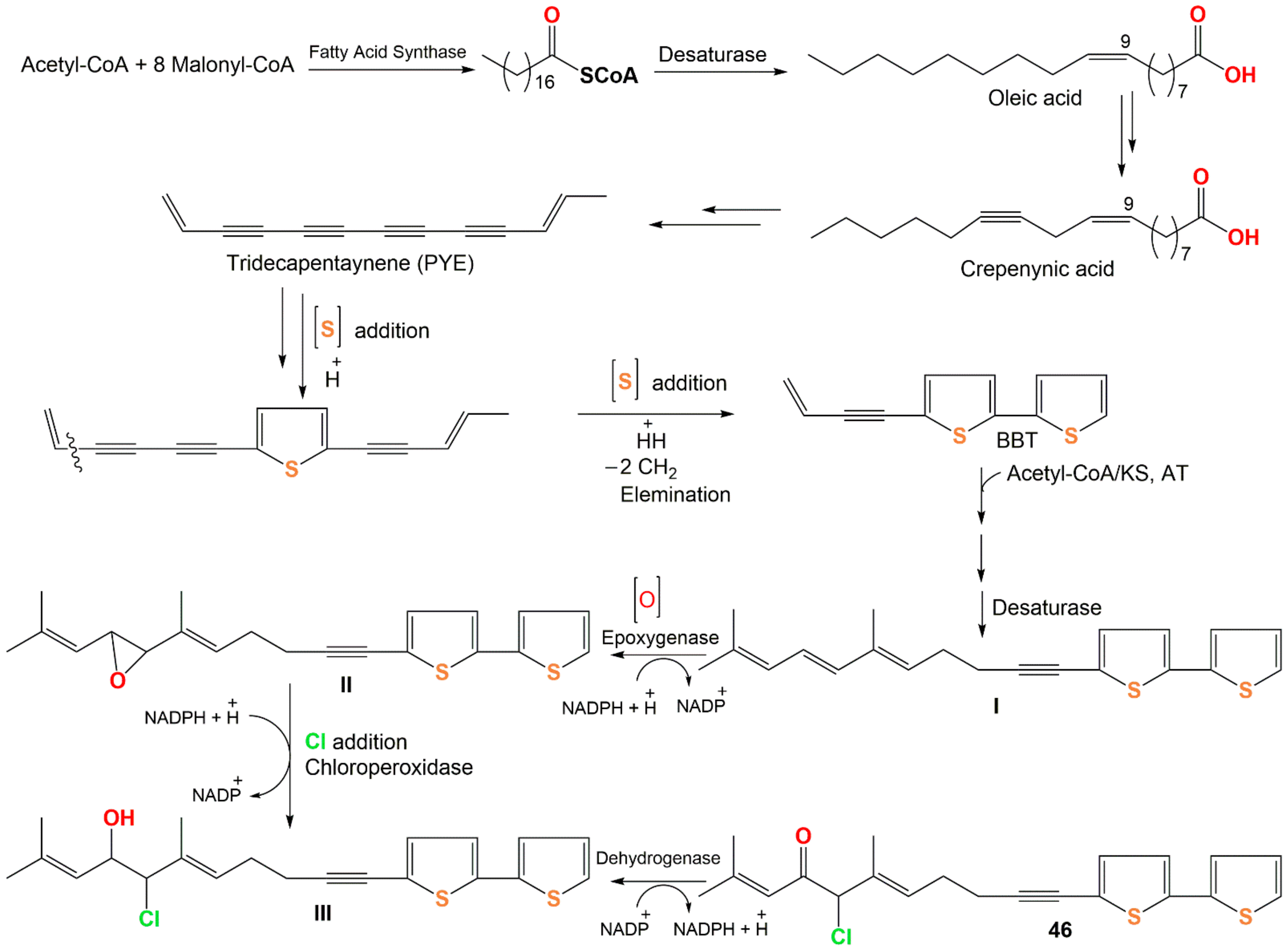
In total, 96 compounds have been listed that have been categorized according to the number of rings into mono-, bi-, ter, and quinque-thiophenes and miscellaneous derivatives. The physical constants and spectral data of the newly reported thiophenes from 2015 to 2021 are included. Further, their possible biosynthetic pathways are illustrated in Scheme 1 and Scheme 2.
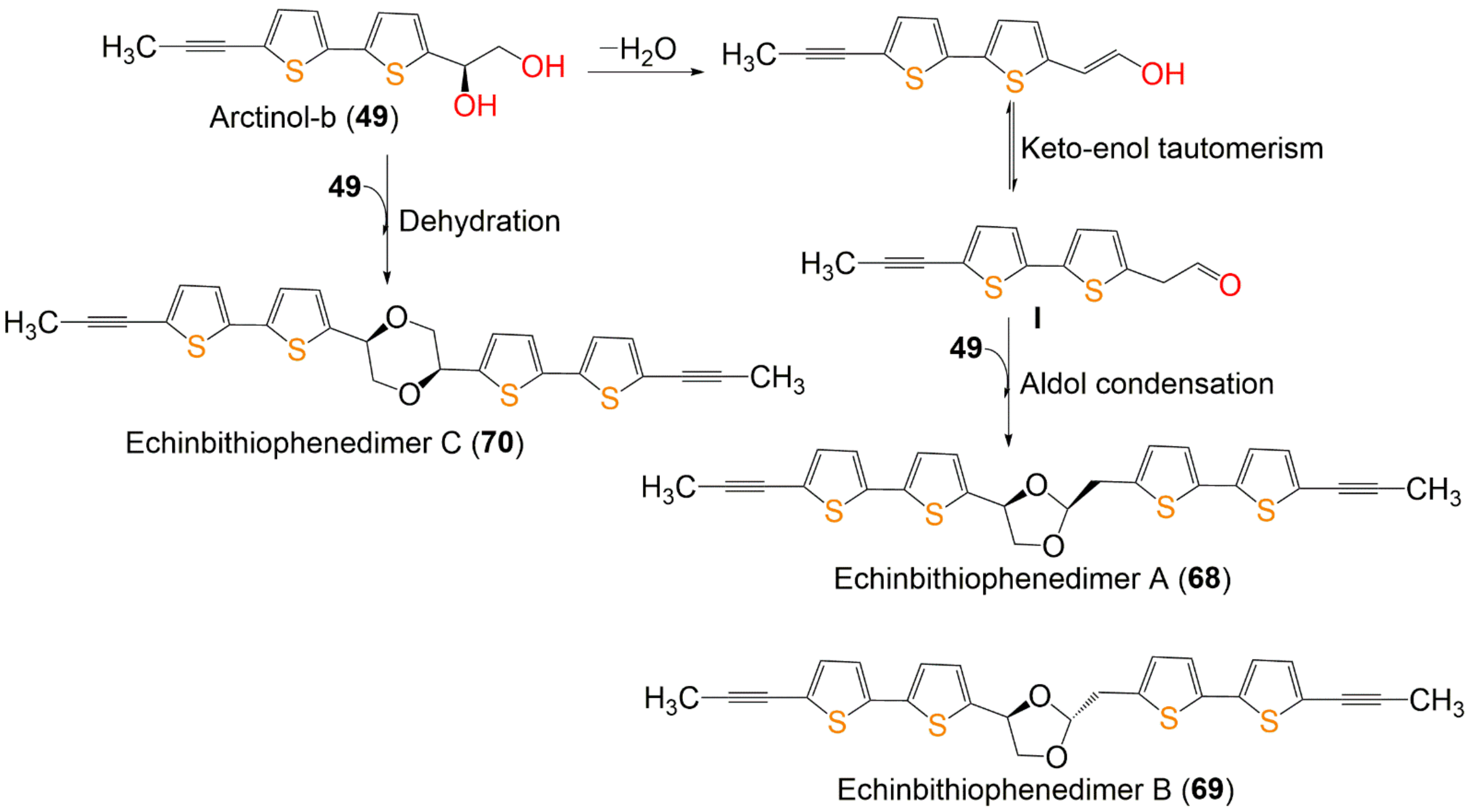
Scheme 1. Proposed biosynthetic pathway of dimeric bithiophenes 68–70 from arctinol-b (49) [17].
Natural proteins (NP) are biologically active molecules with a myriad of structural and functional diversity. They enable the innovative design of synthetic compounds used in medicines, along with many more crucial aspects of molecular medicine, including but not limited to anti-cancer and anti-viral drugs currently in use. Many of them have proved to be incredibly useful in treating a plethora of diseases. Despite its many attributes, the speed and yields of NP-based drug discovery have significantly dropped during the golden period of 1950–1960.
2. Structural Characterization of Thiophenes
The structures of the reported thiophenes were elucidated by various spectral tools such as 1D (one dimensional) (1H and 13C) and 2D NMR (two-dimensional nuclear magnetic resonance spectroscopy) techniques, COSY (homonuclear correlation spectroscopy), HSQC (heteronuclear single quantum coherence), HMBC (heteronuclear multiple bond correlation), and NOESY (nuclear Overhauser effect spectroscopy) combined with other methods (UV (ultraviolet), IR (infra-red), MS (mass spectroscopy), elemental analysis). The relative configuration was determined by NOESY and ROESY (rotating frame Overhauser effect spectroscopy), as well as by [α]D measurement [25]. The exciton coupled circular dichroism (ECCD) analysis and electronic circular dichroism (ECD) calculations were utilized to assess the absolute configuration by comparing the theoretical and experimental CD spectra [16][17][26][27]. Additionally, the determination of the absolute configuration was carried out using Mosher’s method and analyzing chemical shift differences between (S)- and (R)-MTPA [16]. The X-ray structure crystallographic analysis of the crystalline derivatives is another tool utilized for the absolute configuration determination [27]. It was found that some compounds had no names; therefore, they are named here using the AUPAC system for nomenclature. Further, some compounds had the same molecular formulae and structures with different nomenclatures. On the other hand, some metabolites had more than one name.
3. Biosynthesis of Thiophenes
The detailed biosynthesis of thiophenes was discussed previously [8]. In this work, the recently reported biosynthetic pathways was discussed.
Wu et al. reported the biogenetic pathways of dimeric bithiophenes 68–70 (Scheme 1). These compounds had an unparalleled dimeric bithiophene skeleton containing two bithiophene units linked by uncommon cyclic diether units. It was proposed that they may be originated from arctinol-b (49). For 68 and 69, the formation of the 1,3-dioxolane ring may be obtained from an aldol condensation. Firstly, a key intermediate (I) is produced from 49 by dehydration and keto–enol tautomerism. After that, an aldol condensation among 49 and I would give 68 and 69. Additionally, an intermolecular dehydration reaction between two 49 molecules forms the 1,4-dioxane unit to give 70 [17].
Compound 46 originates from oleic acid. The latter is changed into PYE (trideca-3,5,7,9,11-pentayn-l-ene) through successive desaturation steps and shortening of the chain via crepenynic acid [28]. After that, PYE is changed into 5-BBT (5-(but-3-en-1-ynyl)-2,2′-bithiophene) via introducing a sulfur atom and ring formation that is most probably a two-step reaction [29]. Repeated elongation and desaturation of BBT yield I. Then, the double bond epoxidation produces oxirane (epoxy) intermediate II, subsequent addition of chloride by chloroperoxidase forms III, which performs additional dehydrogenation to yield 46 [30][31] (Scheme 2).
4. Biological Activities of Thiophenes
The reported thiophenes were investigated for various bioactivities. In this regard, these metabolites are associated with some types of biological actions, including antimicrobial, antiviral, anti-inflammatory, larvicidal, antioxidant, insecticidal, cytotoxic, and nematicidal effects. The results of the most active metabolites are summarized.
4.1. Anti-Inflammatory Activity
Inflammation is a host body defense mechanism that enables the body to survive during injury or infection and maintains the homeostasis of tissues in noxious conditions [32].
Endogenous NO (nitric oxide) plays a critical role in maintaining the homeostasis of varied cellular functions. NO local concentrations are highly dynamic, as independent enzymatic pathways regulate the synthesis. NO has been shown to modulate inflammation, decreasing the secretion of pro-inflammatory cytokines in human alveolar macrophages challenged with bacterial lipopolysaccharides (LPS) while not altering the basal cytokine levels. Drugs used for managing inflammatory disorders relieve these ailments, but they may have life-threatening consequences [33]. Therefore, there is great enthusiasm in developing new and safe remedies for treating inflammation from natural sources. The reported studies revealed that the anti-inflammatory potential of thiophenes could be due to inhibiting the activation of the NF-κB (nuclear factor-κB) pathway that regulates the expression of pro-inflammatory cytokines and chemokines [34].
The reported studies revealed that thiophenes prohibited TNF-α (tumor necrosis factor-α), IL-6 (interleukin-6), and 5-LOX (5-lipoxygenase), as well as NO production. Thus, their inflammatory potential could be due to the inhibition of NF-κB and NO synthase [35].
Zhou et al. reported that 7 and 8 separated from Artemisia sieversiana exhibited significant anti-neuroinflammatory potential on the LPS-caused NO production in BV-2 murine microglial cells (half-maximal inhibitory concentrations (IC50s) 79.5 and 98.5 µM, respectively), compared to quercetin (IC50 16.3 µM) [36] (Figure 3 and Figure 4).
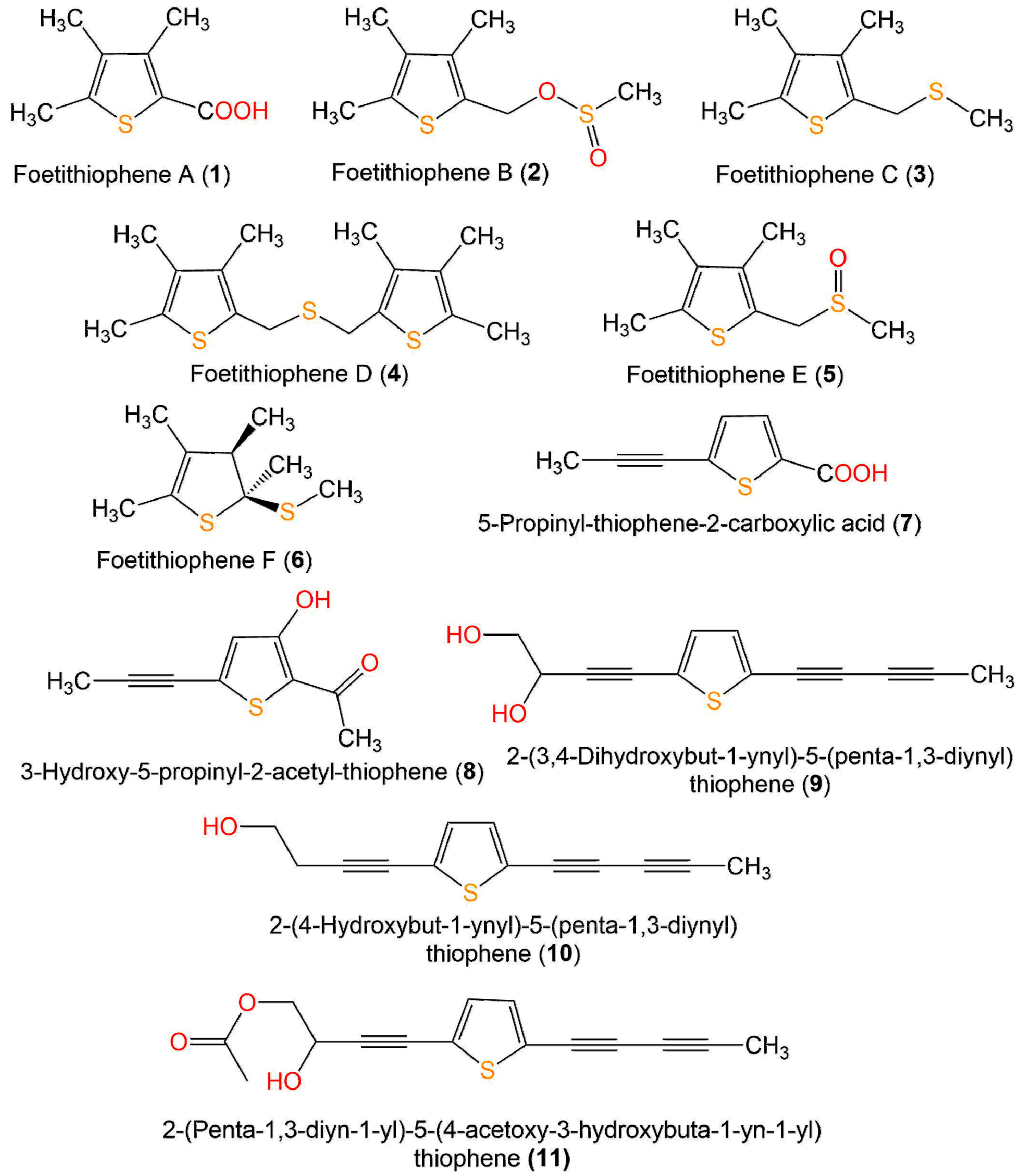
Figure 3. Structures of monothiophenes 1–11.

Figure 4. Structures of monothiophenes 12–21.
In vitro anti-inflammatory assay, compounds 23–26 obtained from Pluchea indica aerial parts possessed significant inhibitory potential toward NO production caused by LPS in RAW 264.7 macrophages at a concentration of 40 µM with % inhibition ranging from 83.4% to 90.1% compared to dexamethasone (62.2%) [37] (Figure 5).

Figure 5. Structures of monothiophenes 22–31.
On the other side, the two new thiophene polyacetylene glycosides, atracthioenynesides A (29) and B (30) isolated from Atractylodes lancea rhizomes did not show any activity in LPS-induced NO production in BV2 cells [26].
A new bithiophene, 32, along with 16 formerly separated thiophenes, 9, 10, 33–45, and 75, were purified from Echinops grijisii roots EtOAc-soluble fraction of the MeOH extract using SiO2 CC (column chromatography) eluted with n-hexane-EtOAc gradient as well as HPLC and identified by IR, UV, NMR, and HRESIMS spectroscopy [38] (Figure 6 and Figure 7).
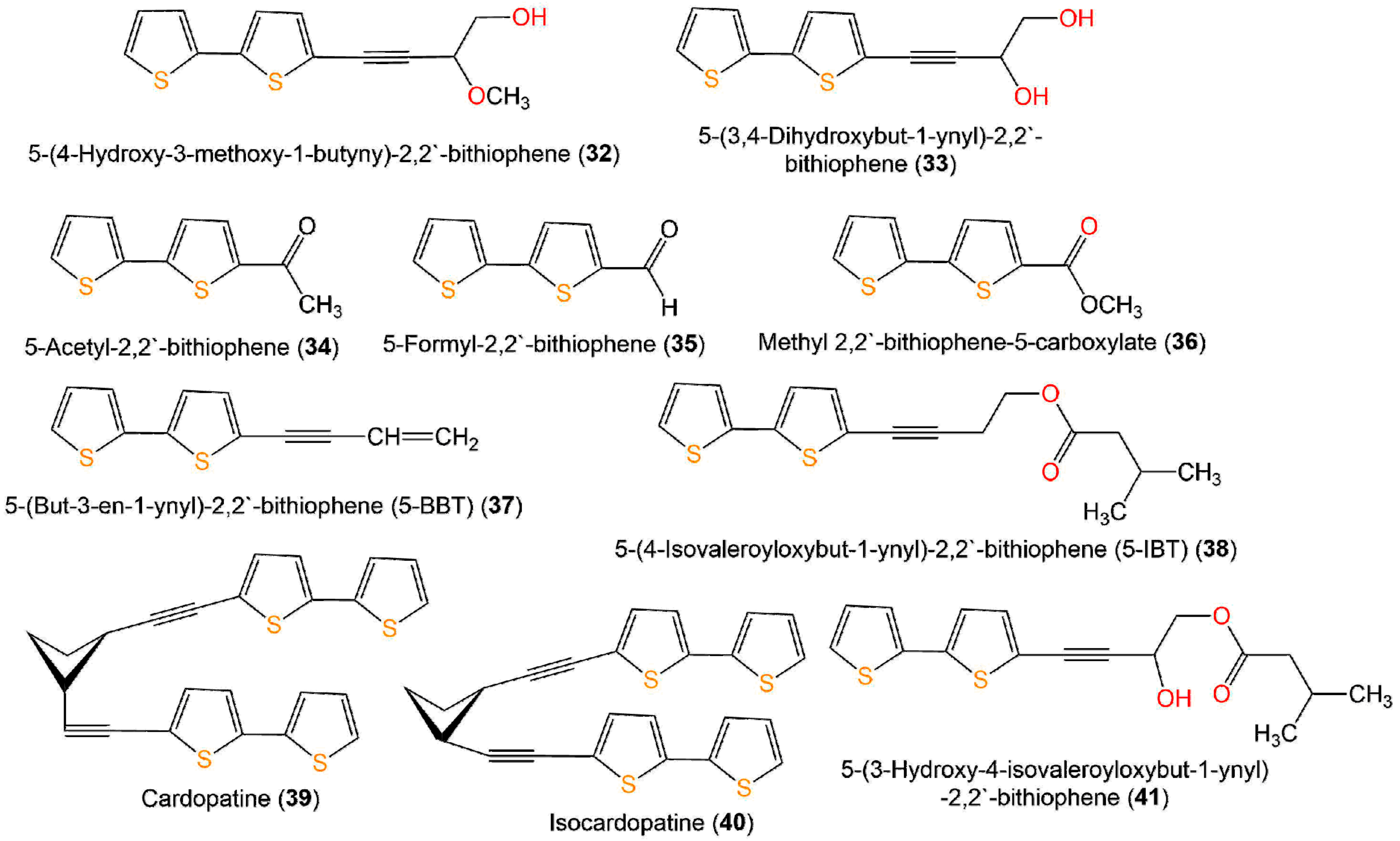
Figure 6. Structures of compounds 32–41.
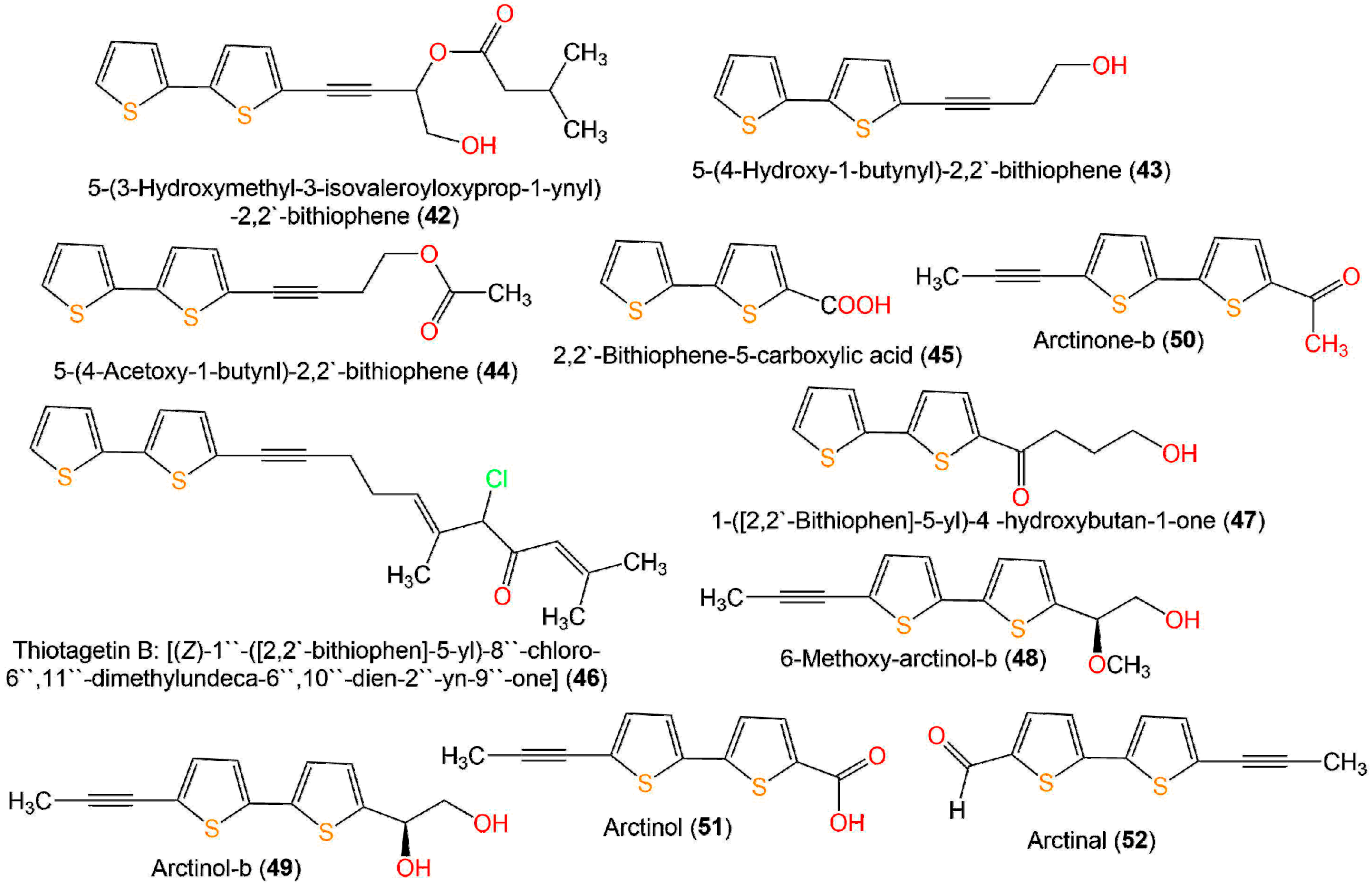
Figure 7. Structures of bithiophenes 42–52.
These compounds were assessed for anti-inflammatory activity versus RAW 264.7 cells. Only 9, 33, and 43 (IC50s 2.5, 20.0, and 6.7 µg/mL, respectively) exhibited significant in vitro anti-inflammatory potential toward LPS-boosted NO production in RAW 264.7 cells compared to indomethacin (IC50 65.4 µg/mL) in the colorimetric assay [38]. Zhang et al. purified three new derivatives: rupestrienes A–C (86, 27, and 28), Artemisia rupestris EtOH extract by SiO2, RP-18, and Sephadex CC. Rupestrienes B and C (27 and 28) displayed significant inhibitory potential (IC50 8.5 and 5.3 μM, respectively) toward LPS-caused NO production in BV-2 microglial cells, compared to quercetin (IC50 4.3 μM), 86 was weakly active (IC50 20.3 μM) [39]. Jin et al. assessed the inhibitory potential of 19, 20, 48, 49, 51, and 55 toward NO production boosted by LPS in RAW 264.7 cells. Only 19, 20, 48, and 49 exhibited moderate inhibitory potential (IC50 12.8–48.7 µM), compared to indomethacin and aminoguanidine (IC50s 13.2 and 24.2 µM, respectively). On the other side, 51 and 55 did not have any activity (IC50 ˃100 µM) [25]. The structure–activity relationship revealed that the monothiophenes with two acetylene units were more potent than bithiophenes with one acetylene unit. The existence of the Δ10,11 cis double bond and 1,2-diol at C-5 enhanced the inhibitory activity [25].
Compounds 43, 46, and 76 separated from aerial parts of Tagetes minuta significantly decreased NFκB p65, TNF-α, and IL-6 compared to indomethacin in the ELISA (enzyme-linked immunosorbent assay) [29]. In 2020, Ibrahim et al. reported that 43 and 76 isolated T. minuta displayed moderate anti-inflammatory potential (IC50 41.82 and 26.18 µM, respectively) in the 5-LOX colorimetric assay in comparison to indomethacin (IC50 0.89 µM) [40].
4.2. Cytotoxic Activity
Cancer is a crucial cause of death globally, accounting for ≈10 million deaths in 2020 [41][42]. There are many available medications for treating various types of cancer. However, none of them are entirely safe and effective. Many of the reported thiophenes have been assessed for cytotoxic effectiveness toward various cancer cell lines.
Four new derivatives, foetithiophenes C-F (3–6), along with foetithiophenes A (1) and B (2), were obtained from MeOH extract of Ferula foetida roots using SiO2 CC and RP-HPLC. Unfortunately, they showed no cytotoxic capacity (IC50 ˃100 µmM) versus K562 and MCF-7 cell lines in the Alamar Blue assay [43].
Additionally, 9 had more promising cytotoxic potential (IC50 21.09 µM) than doxorubicin (IC50 195.12 µM) against CEM/ADR5000 (human T-cell lymphoblast-like cell line). However, it was weakly active toward CCRF-CEM (human leukemic cell line, IC50 46.96 µM) in the resazurin reduction cytotoxic assay [44].
Compounds 11, 18, and 22 isolated from Pluchea indica aerial parts were assayed for inhibitory potential on coumarin 7-hydroxylation induced by CYP2A6 (cytochrome P450 2A6) and CYP2A13 (cytochrome P450 2A13) enzymes, using enzymatic reconstitution assay [45]. The human liver cytochrome P450 (CYP) 2A13 and 2A6 enzymes had a crucial function in nicotine metabolism and the activation of tobacco-specific nitrosamine carcinogens. Their prohibition could represent a strategy for smoking abstinence and decreasing risks of lung cancer and respiratory complaints. It was found that 18, 11, and 22 irreversibly prohibited CYP2A6- and CYP2A13-induced coumarin 7-hydroxylation (IC50 values 3.90 and 2.40 µM, respectively, for 18; IC50 6.43 and 6.18 µM, respectively for 11, and IC50 4.44 and 2.94 µM, respectively for 22). These metabolites could aid in smoking stoppage and lessened risks of lung cancer and respiratory illnesses [45].
Xu et al. reported that the treatment of SW620 (human colon cancer) cells with PYDDT (2-(pro-1-ynyl)-5-(5,6-dihydroxypenta-1,3-diynyl) thiophene) (18) led to the induction of mitochondrial-mediated apoptosis that was featured by cleavage of PARP (poly ADP ribose polymerase), activating caspase-3 and 9, the release of cytochrome c from mitochondria, mitochondrial membrane potential loss, Bcl-2 (B-cell lymphoma 2) downregulation, and Bax mitochondrial translocation. A mechanism study revealed that PYDDT induced SW620 apoptosis through a JNK (c-Jun N-terminal kinase)/ROS (reactive oxygen species)-mediated mitochondrial pathway [46].
Ecliprostins A–C (65–67) new thiophene derivatives were separated from Eclipta prostrata. In contrast, ecliprostins A (65) and B (66) featured a bithiophenyl acetylenic skeleton, incorporating an isovalerate unit, whereas ecliprostin C (67) was a dimer of 65. They exerted no noticeable cytotoxicity versus Hela and MDA-MB-231 cell lines (Conc. 30 µM) [18].
Compounds 33, 75–78, and 82 were purified from the EtOH extract of Eclipta prostrata aerial parts by SiO2 CC (silica gel column chromatography) and purified using a reversed-phase CC. In the MTT assay, 77 exhibited the most potent cytotoxicity on SKOV3 cells (IC50 7.73 µM) than cisplatin (IC50 11.25 µM). The terthiopenes 75, 76, and 82 showed significant cytotoxicity (IC50 values ranging from 24.57 to 77.23 µM). However, 33 and 78 were ineffective (IC50 values ˃ 100 µM) [47].
Additionally, Preya et al. reported that 77 isolated Eclipta prostrata was a more potent cell growth inhibitor (IC50s 0.20–18.82 µM) than cisplatin (IC50 10.80 to 43.05 µM) toward a panel of human ovarian cancer cell lines; OVCAR3, SKOV3, A2780, and ES2 in the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. It caused changes in S phase-linked proteins (cyclins A and D2 and cyclin-dependent kinase 2) and induced an intracellular increase in ROS that increased the levels of p-H2AX (H2A histone family member X), resulting in DNA (deoxyribonucleic acid) damage [14][20]. A mechanism study indicated that 77 caused S-phase cell cycle arrest by inducing ROS stress and DNA damage. Therefore, 77 could be a potential therapeutic lead for treating ovarian cancer.
Sibiricumthionol (84) and (+)-xanthienopyran (85) were purified from Xanthium sibiricum fruits extract using SiO2, RP-18 (reversed phase-18), and HPLC (high-performance liquid chromatography) that were characterized by spectroscopic, X-ray, and ECCD analyses, as well as ECD calculations. These metabolites were inactive (IC50 ˃10 µM) toward HCT-116, BGC-823, HepG2, NCI-H1650, and A2780 cell lines in the MTT assay [27].
Compounds 76, 77, and 79–81 isolated from Eclipta prostrate showed prominent cytotoxic effectiveness toward Hec1A (IC50 ranging from 0.38 to 129.85 µM) and Ishikawa (IC50 ranging from 0.35 to 9.68 µM) cells compared to cisplatin (IC50 120.4 and 10.11 µM, respectively). Notably, 77 had a potent effect on Ishikawa and Hec1A cells (IC50 0.35 and 0.38 µM, respectively) [26][48]. The inhibitory effect of 77 was mediated by the induction of apoptosis, triggering caspase activation and cytochrome c release into the cytosol. Additionally, it increased the ROS intracellular level and decreased GSH (glutathione). Therefore, its apoptotic effect was attributed to the generation of reactive oxygen species via NADPH (nicotinamide adenine dinucleotide phosphate) oxidase in human endometrial cancer cells [48].
Thiotagetin A (83) purified from Tagetes minuta possessed cytotoxic capacity versus MCF-7 and KB (ED50s 3.88 and 2.03 μg/mL, respectively), compared to adriamycin (0.07 and 0.26 μg/mL, respectively) in the MTT assay [41].
4.3. Antimicrobial Activity
Infectious diseases continue to be a serious worldwide health concern. Multidrug-resistant (MDR) pathogens significantly increased morbidity and mortality rates [49]. The continuous emergence of MDR pathogens drastically reduced the efficacy of the utilized antibiotics resulting in a growth rate of therapeutic failure [50]. Accordingly, new and effective antimicrobial agents to tackle microbial infections are needed [51].
Chitsazian-Yazdi et al. assayed the antimicrobial activity of 1–6 in broth microdilution method toward B. cereus PTCC-1247, C. albicans ATCC-10231, and E. coli ATCC-8739. Whereas only 6 displayed the most potent potential (MIC 50 µg/mL) against B. cereus, compared to gentamicin (MIC 10 µg/mL) [43].
Mbaveng et al. purified 9 from the CH2Cl2 fraction of Echinops giganteus roots. It showed moderate and selective activities against E. coli ATCC-8739, E. aerogenes ATCC-13048 and -EA27, K. pneumonia ATCC11296, P. stuartii ATCC29916, E. cloacae BM47, and P. aeruginosa PA01 (MIC <100 µg/mL) in the rapid INT (p-iodonitrotetrazolium) chloride assay [52].
In 2017, Postigo et al. reported the separation and structural elucidation of 37, 43, 44, and 75 the from n-hexane extract of Porophyllum obscurum by preparative CTL (centrifugal thin layer) and TL (thin-layer) chromatography that were assayed for their fungicidal potential against C. albicans ATCC-10231 and 25 clinical strains of Candida spp. isolates as causative agents of oropharyngeal candidiasis using broth microdilution. They exhibited fungicidal effectiveness with minimum fungicidal concentrations (MFC) ranging from 0.24 to 7.81 μg/mL under UV-A irradiation, whereas 32 with (MFC 0.24 μg/mL) and 43 with (MFC 3.90 μg/mL) were the most active metabolites [53]. In 2019, Postigo et al. evaluated their photoinactivation towards C. albicans in parallel under darkness and light conditions. The results revealed that these thiophenes exhibited the highest potential under normal-light/oxygen atmosphere (MFCs ranged from 0.24 to 7.81 μg/mL). However, their effects decreased >200 times (MFCs ranged from 7.81 to 250 μg/mL) with low-oxygen conditions. On the other hand, all tested thiophenes had no antifungal potential in darkness under both oxygen conditions (MFC > 250 μg/mL). It was found that 75 was the most active photosensitizer and was the only one that generated a single oxygen at MFC. Furthermore, it did not elevate sensitivities to oxidative and osmotic stressors and did not produce leakage or apoptosis [54]. Therefore, their antifungal mechanism was proposed to be photodynamic, considering that the absence of oxygen had a passive effect on the antifungal photosensitivity capacity. Therefore, these features could encourage further assessments to confirm their potential application as photosensitizers in photodynamic antimicrobial therapy toward fungal infections [54].
Li et al. performed a broth microdilution assay for evaluating the antimicrobial potential of 7, 9, 33, 34, 43, 45, 47, 49, 52–54, and 57–60 (Figure 8) isolated from E. ritro versus E. coli, S. aureus, and C. albicans. Compounds 43, 49, 53, and 58 exhibited the same antibacterial activity toward S. aureus as levofloxacin (MIC (minimum inhibitory concentration) 8 µg/mL). Additionally, 43, 49, 52, 53, and 58 possessed activity against E. coli (MIC values of 32–64 µg/mL). On the other side, 43, 49, and 58 displayed antifungal potential toward C. albicans (MIC values of 32–64 µg/mL) that was similar or two-fold more active than levofloxacin (MIC 64 µg/mL) [55].
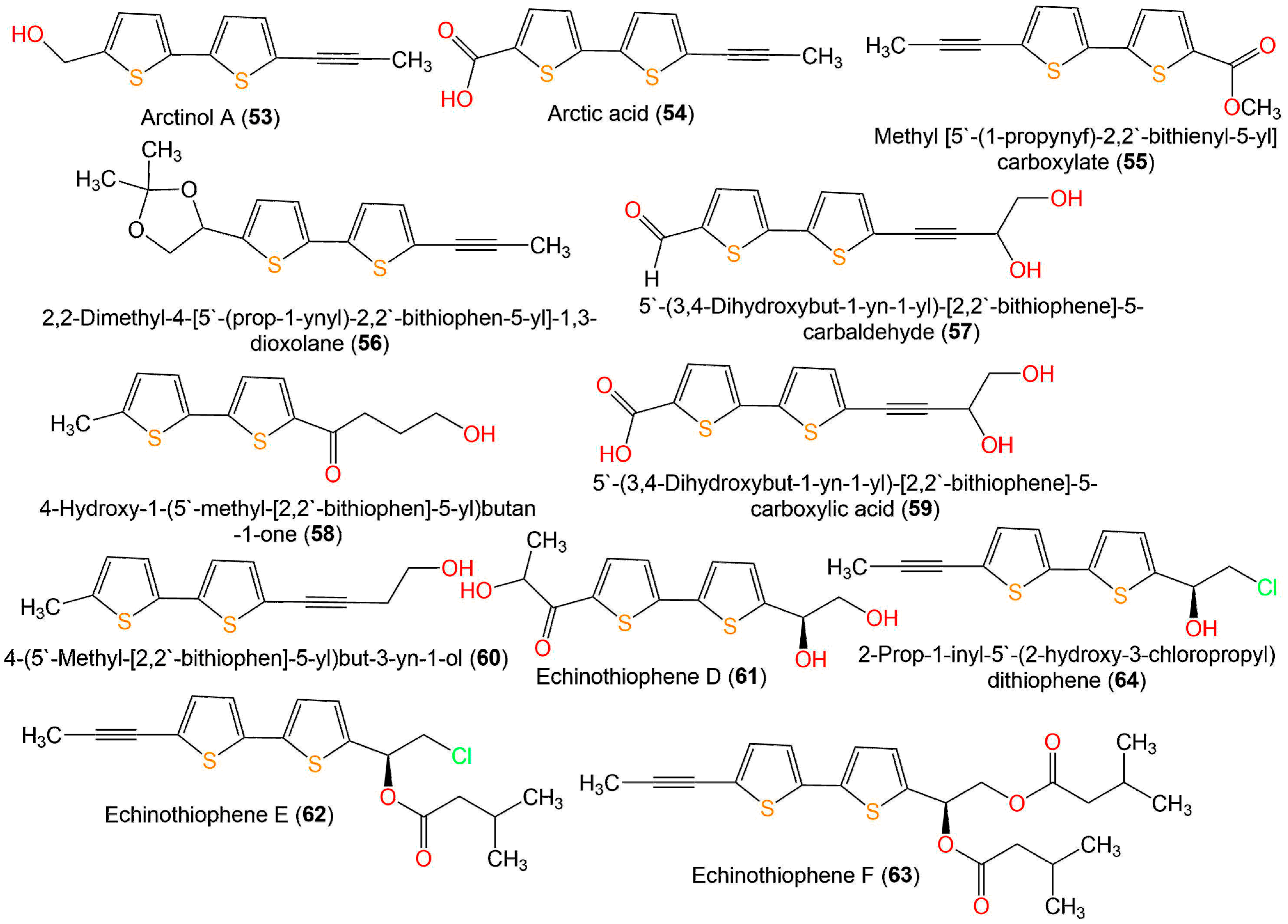
Figure 8. Structures of bithiophenes 53–63.
Liu et al. reported that 15, 16, 48, 51, 61, 62, and 64 possessed equivalent or better antifungal capacities toward Fusarium solani, Colletotrichum gloeosporioides, F. oxysporum f. sp. vasinfectum, Phytophthora infestans, Alternaria alternata, and F. oxysporum f. sp. niveum compared to carbendazim, whereas 17, 48, 50, 52, and 63 had weak antifungal potential (MICs from 32 to >256 µg/mL). It is noteworthy that 15 (MICs 4 and 8 µg/mL, respectively) had elevated inhibitory capacity toward A. alternata and F. oxysporum f. sp. niveum compared to 16, 17, and 62 (MICs from 8 to >256 µg/mL), indicating that acylation weakened the activity. Further, the effect of 15 and 16 versus all fungi was more than that of 17, suggesting that chlorine could enhance activity [19].
Compounds 65–67 showed moderate growth inhibition against S. aureus (MICs 25.0, 6.25, and 25.0 µM, respectively) in the broth microdilution assay, compared to penicillin (MIC 0.156 µM) [18], whilst they did not have significant activity toward Vibrio vulnificus and E. coli [18].
Echinbithiophenedimers A–C (68–70) novel dimeric bithiophenes, besides 37 and 49, were separated from Echinops latifolius using SiO2, Sephadex CC, and PTLC (Figure 9). Their antifungal potential against soil-borne fungi; Pyricularia oryzae, Alternaria alternata, Colletotrichum gloeosporioides, Fusarium oxysporum, and Phytophthora infestans were assessed in light and dark by the micro-broth dilution method. Compounds 68–70 had significant antifungal capacities toward P. oryzae and A. alternata (MICs 8–16 µg/mL), whereas 70 (MIC 8 µg/mL) displayed better antifungal potential toward A. alternata than carbendazim (MIC 16 µg/mL). Additionally, they revealed more antifungal potential (MIC 28 µg/mL) against P. infestans than carbendazim (MIC 256 µg/mL). It was found that an increased thiophene rings′ number bettered the activity [17].

Figure 9. Structures of bithiophenes 64–72.
Yu et al. purified two new thiophenes derivatives, 31 and 71, together with 9, 33, 48, 49, 71–73, 77, and 82 from Eclipta prostrata by SiO2, Sephadex CC, and RP-HPLC [56]. Only 77 and 82 exerted mild antibacterial potential toward S. aureus (MIC 25 µM) in the broth microdilution method, compared to penicillin G (MIC 0.156 µM) (Figure 10) [56].
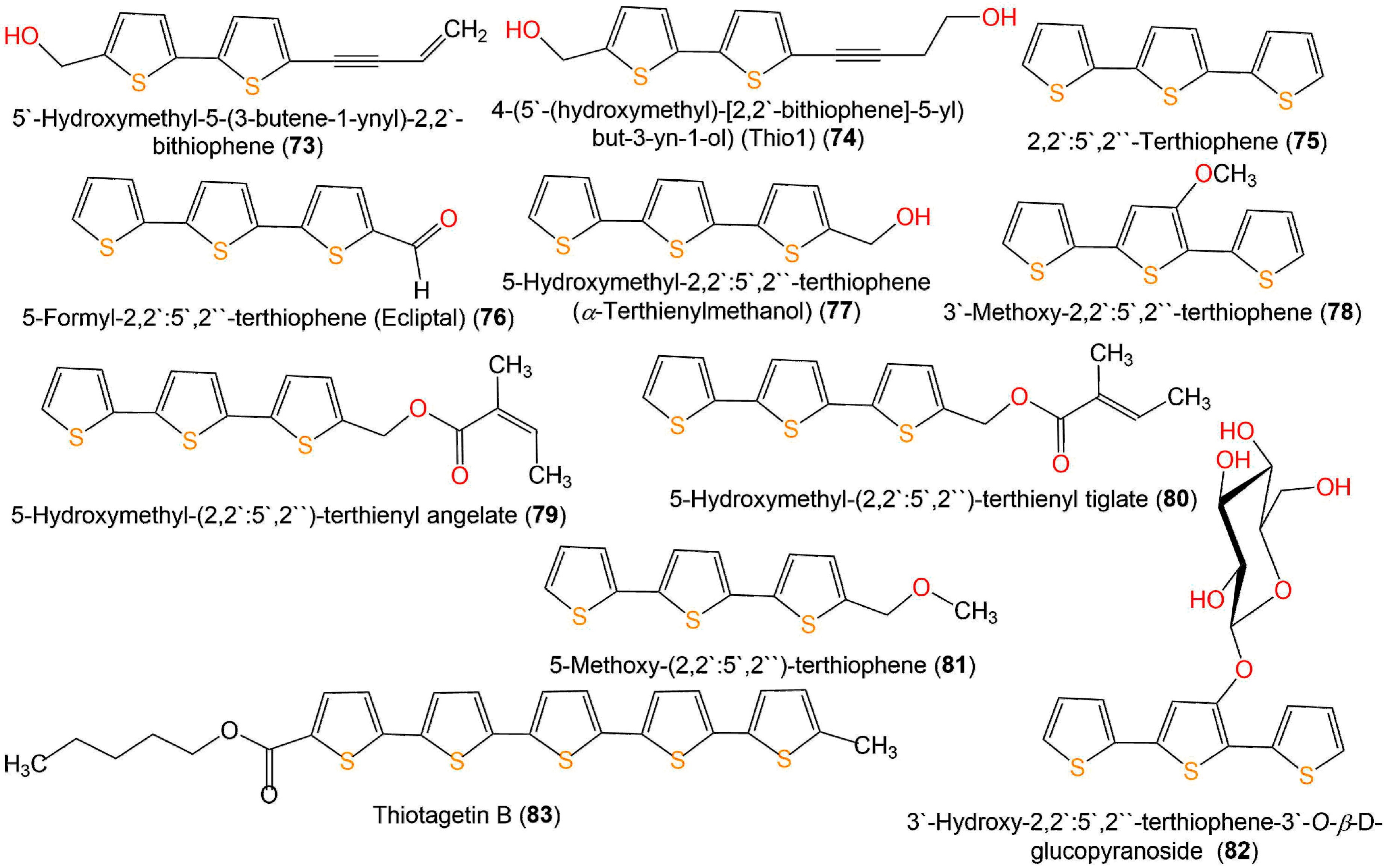
Figure 10. Structures of bithiophenes (73 and 74), terthiophenes (75–82), and quinquethiophene 83.
Compound 87 was biosynthesized using endolithic Streptomyces sp. AL51. This compound had remarkable antibacterial potential versus both Gram-positive and -negative bacteria in the microplate broth-dilution method. It displayed higher activity than penicillin against Gram-positive S. aureus, B. subtilis, E. coli, and Klebsiella pneumonia with MIC/MBC (minimum bactericidal concentration) 0.2/2.0, 0.25/0.5, 4.0/8.0, and 4.0/16.0 µg/mL, respectively, compared to penicillin (MIC/MBC 32.0/64.0, 0.5/4.0, 4.0/16.0, and 16.0/64.0 µg/mL, respectively) [51].
Cao et al. purified 88 from the culture broth of the marine-derived actinomycete Streptomyces sp. G278 selectively prohibited Enterococcus faecalis equal to streptomycin (MIC 256 μg/mL) [57].
Six novel thiophene-furan-carboxylic acids, 89–94, were isolated from the soil-derived fungus Penicillium sp. Sb62, representing the first class of natural furan-carboxylic acids having a thiophene moiety (Figure 11). They possessed antimicrobial capacities versus E. coli, S. aureus, and C. albicans with MICs 0.9–7.0, 1.7–3.5, and 3.3–7.0 μg/mL, respectively, in the broth microdilution assay. It was observed that the absence of methoxy or a hydroxy substituent on the side chain enhanced the activity similar to 89 and 90, and the configurations of the methoxy or hydroxy groups on the side chain had a little effect as in 91, 92, 93, and 94 [16].

Figure 11. Structures of miscellaneous thiophenes 84–96.
4.4. Antimalarial Activity
Malaria represents a significant parasitic disease worldwide, which is accountable for the death of at least half a million people yearly [58]. Globally, the estimated malaria cases in 2020 are 241 million in 85 malaria-endemic countries [59]. There is currently a vast augmentation of resistance to the available antimalarial drugs, which necessitates the search to pinpoint new drugs to combat malaria [60].
Bitew et al. evaluated the antimalarial activity of 9 and 14 isolated from CH2Cl2 fraction of Echinops hoehnelii roots utilizing the standard suppressive method in Plasmodium berghei-affected mice. Compounds 9 and 14 at 50 and 100 mg/kg concentrations decreased parasitemia levels by 43.2% and 50.2% and 18.8% and 32.7%, respectively, compared to chloroquine. It was suggested that the ester functional group produced a two-fold decrease in the activity as in 14 [61].
4.5. Larvicidal Activity
Currently used larvicides are synthetic pesticides with high toxic effects on humans and other non-targeted organisms. Several reports revealed that thiophenes demonstrated toxic effect toward insects, especially larval mosquitoes. It was proposed that thiophenes showed the promising possibility to be set as natural larvicides for controlling mosquitoes.
Zhao et al. reported that E. grijsii essential oil exhibited larvicidal potential versus the fourth instar larvae of Anopheles sinensis, Culex pipiens pallens, and Aedes albopictus (LC50s (lethal concentrations 50%) s 3.43, 1.47, and 2.65 µg/mL, respectively) in the larval mortality bioassay compared to rotenone. Further, the purified metabolites; 5-BBT (5-(but-3-en-1-ynyl)-2,2′-bithiophene) (37), 5-IBT (5-(4-isovaleroyloxybut-1-ynyl)-2,2′-bithiophene) (38), and α-T (α-terthienyl) (75) possessed remarkable larvicidal effectiveness (LC50 0.34, 0.45, and 1.41 µg/mL, respectively for Ae. albopictus, LC50 1.36, 5.36, and 1.79 µg/mL, respectively for An. sinensis, and LC50 0.12, 0.33, and 1.38 µg/mL, respectively for C. pipiens pallens) compared to rotenone (LC50 3.75, 1.25, and 1.88 µg/mL, respectively) [62].
4.6. Nematicidal Activity
Nematodes and plant pathogenic fungi cause diseases that can lessen the yield and quality of several crops [63]. Chemical control utilizing synthetic-produced pesticides is a commonly used way to manage these diseases. The possible imperilment of synthetic chemicals toward non-target organisms and pesticide resistance rationalized the development of eco-friendly and safe pesticides [64]. Discovering efficient and less toxic natural pesticides has given rise to a top preference in the contemporaneous pesticide industry [65].
Compounds 15, 16, 48, 50, 52, 61, 62, and 64 showed more potent nematicidal effect toward J2s (second-stage juveniles) of Meloidogyne incognita (LC50 values ranging from 0.42 to 8.28 μg/mL in light and from 0.86 to 9.23 μg/mL in dark) than abamectin (LC50 values 9.38 μg/mL in dark and 8.73 μg/mL in light). Noticeably, 61 and 64 possessed better dark potential compared to their light potential than control. Particularly, 64 was the most powerful metabolite against J2s (LC50 values 0.91 and 0.86 μg/mL, under light and dark, respectively) [13]. Compounds 48, 49, 51, and 61–64 were regarded as non-phototoxic metabolites. It was found that the thiophene unit was fundamental for the activity. However, an increase in the number of acetylenes and chlorine enhanced the effect [13][19]. Compounds 68–70 were evaluated for their nematicidal potential toward the J2s of Meloidogyne incognita under dark and light conditions in nematode mortality bioassays. They showed potent nematicidal potential (LC50 9.39–18.17 µg/mL/dark and 8.73–16.53 µg/mL/light) compared to ethoprophos (LC50 31.94 µg/mL/dark and 36.15 µg/mL/light). However, they had weaker nematicidal influences than α-terthienyl (phototoxic thiophene), suggesting that they were non-phototoxic. Furthermore, 70 exhibited more powerful activity (LC50 8.73 and 9.39 µg/mL under light and dark, respectively) than its monomeric bithiophene 49, revealing that the dimeric bithiophene framework with a 1,4-dioxane moiety in 70 enhanced the nematicidal potential [17].
Compound 74 previously reported from Tagetes patula aerial parts was synthesized by Politi et al. It had a marked in vitro anthelmintic effect toward Haemonchus contortus, exhibiting 100% efficacy in the larval development and egg hatch tests with EC50 (effective concentration 50%) 0.3243 mg/mL and 0.1731 mg/mL, respectively, compared to levamisole (EC50 1.88 mg/mL) [66].
4.7. Antioxidant and Anti-Influenza Activities
Compounds 43, 46, and 76 exhibited moderate antioxidant potential with % DPPH scavenging activity ranging from 41.87 to 45.17 at 100 µM [29].
Two new thiophene derivatives, rupestriene D (95) and rupestriene E (96), along with rupestriene A (86) isolated from the whole plants of Artemisia rupestris using SiO2 CC and RP-HPLC. They exhibited neuraminidase inhibitory potential with IC50 values ranging from 351.15 to 986.54 µM in the fluorescence-based assay compared to oseltamivir acid (IC50 77.91 µM). Compounds 86 and 96 were more potent than 95, indicating that a free OH group at the C-3 side chain might enhance the activity [15].
This entry is adapted from the peer-reviewed paper 10.3390/plants11040539
References
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L.R.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Heterocyclic anticancer compounds: Recent advances and the paradigm shift towards the use of nanomedicine’s toolbox. Molecules 2015, 20, 16852–16891.
- Tilby, M.J.; Willis, M.C. How do we address neglected sulfur pharmacophores in drug discovery? Expert Opin. Drug Discov. 2021, 16, 1227–1231.
- Ward, N.P.; DeNicola, G.M. Sulfur metabolism and its contribution to malignancy. Cell. Nutr. Util. Cancer 2019, 347, 39–103.
- Francioso, A.; Baseggio Conrado, A.; Mosca, L.; Fontana, M. Chemistry and biochemistry of sulfur natural compounds: Key intermediates of metabolism and redox biology. Oxid. Med. Cell Longev. 2020, 2020, 8294158.
- Rakesh, K.P.; Wang, S.M.; Leng, J.; Ravindar, L.; Asiri, A.M.; Marwani, H.M.; Qin, H.L. Recent development of sulfonyl or sulfonamide hybrids as potential anticancer agents: A key review. Anti-Cancer Agents. Med. Chem. 2018, 18, 488–505.
- McGrath, N.A.; Brichacek, M.; Njardarson, J.T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010, 87, 1348–1349.
- Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar. Life Sci. Technol. 2021, 3, 488–518.
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Mohamed, G.A. Naturally occurring thiophenes: Isolation, purification, structural elucidation, and bioactivities. Phytochem. Rev. 2016, 15, 197–220.
- Shah, R.; Verma, P.K. Therapeutic importance of synthetic thiophene. Chem. Cent. J. 2018, 12, 137.
- Keri, R.S.; Chand, K.; Budagumpi, S.; Balappa Somappa, S.; Patil, S.A.; Nagaraja, B.M. An overview of benzothiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033.
- Gramec, D.; Masic, L.P.; Sollner Dolenc, M. Bioactivation potential of thiophene-containing drugs. Chem. Res. Toxicol. 2014, 27, 1344–1358.
- Joshi, E.M.; Heasley, B.H.; Chordia, M.D.; Macdonald, T.L. In vitro metabolism of 2-acetylbenzothiophene: Relevance to Zileuton Hepatotoxicity. Chem. Res. Toxicol. 2004, 17, 137–143.
- Gil, A.; Ghersa, C.M.; Perelman, S. Root thiophenes in Tagetes minuta L. accessions from Argentina: Genetic and environmental contribution to changes in concentration and composition. Biochem. Syst. Ecol. 2002, 30, 1–13.
- Champagne, D.E.; Arnason, J.T.; Philogene, B.J.R.; Campbell, G.; McLachlan, D.G. Photosensitization and feeding deterrence of Euxoa messoria (Lepidoptera: Noctuiidae) by α-terthienyl, a naturally occurring thiophene from the Asteraceae. Experientia 1984, 40, 577–578.
- Cao, Y.; Zang, Y.; Huang, X.; Cheng, Z. Chemical constituents from Artemisia rupestris and their neuraminidase inhibitory activity. Nat. Prod. Res. 2021, 35, 1775–1782.
- Chang, J.L.; Xu, H.Z.; Zhou, J.; Zhou, M.; Zhang, X.; Guo, Y.; Ruan, H.L. Antimicrobial furancarboxylic acids from a Penicillium sp. J. Nat. Prod. 2020, 83, 3606–3613.
- Wu, H.B.; Wu, H.B.; Kuang, M.S.; Lan, H.P.; Wen, Y.X.; Liu, T.T. Novel bithiophene dimers from Echinops latifolius as potential antifungal and nematicidal agents. J. Agric. Food Chem. 2020, 68, 11939–11945.
- Yu, S.J.; Yu, J.H.; He, F.; Bao, J.; Zhang, J.S.; Wang, Y.Y.; Zhang, H. New antibacterial thiophenes from Eclipta prostrata. Fitoterapia 2020, 142, 104471.
- Liu, T.; Wu, H.; Jiang, H.; Zhang, L.; Zhang, Y.; Mao, L. Thiophenes from Echinops grijsii as a preliminary approach to control disease complex of root-knot nematodes and soil-borne fungi: Isolation, activities, and structure-nonphototoxic activity relationship analysis. J. Agric. Food Chem. 2019, 67, 6160–6168.
- Preya, U.H.; Lee, K.T.; Kim, N.J.; Lee, J.Y.; Jang, D.S.; Choi, J.H. The natural terthiophene α-terthienylmethanol induces S phase cell cycle arrest of human ovarian cancer cells via the generation of ROS stress. Chem. Biol. Interact. 2017, 272, 72–79.
- Da Cruz, R.M.D.; Mendonça-Junior, F.J.B.; de Mélo, N.B.; Scotti, L.; de Araújo, R.S.A.; de Almeida, R.N.; de Moura, R.O. Thiophene-based compounds with potential anti-inflammatory activity. Pharmaceuticals 2021, 14, 692.
- Caballero, R.; Cohen, B.; Gutiérrez, M. Thiophene-based covalent organic frameworks: Synthesis, photophysics and light-driven applications. Molecules 2021, 26, 7666.
- Bhilare, N.V.; Auti, P.B.; Marulkar, V.S.; Pise, V.J. Diverse thiophenes as scaffolds in anti-cancer drug development: A concise review. Mini-Rev. Med. Chem. 2021, 2, 217–232.
- Abedinifar, F.; Babazadeh Rezaei, E.; Biglar, M.; Larijani, B.; Hamedifar, H.; Ansari, S.; Mahdavi, M. Recent strategies in the synthesis of thiophene derivatives: Highlights from the 2012–2020 literature. Mol. Divers. 2021, 25, 2571–2604.
- Jin, Q.; Lee, J.W.; Jang, H.; Choi, J.E.; Kim, H.S.; Lee, D.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Dimeric sesquiterpene and thiophenes from the roots of Echinops latifolius. Bioorg. Med. Chem. Lett. 2016, 26, 5995–5998.
- Feng, Z.M.; Xu, K.; Wang, W.; Du, N.; Zhang, J.H.; Yang, Y.N.; Jiang, J.S.; Zhang, P.C. Two new thiophene polyacetylene glycosides from Atractylodes lancea. J. Asian Nat. Prod. Res. 2018, 20, 531–537.
- Shi, Y.S.; Li, L.; Liu, Y.B.; Ma, S.G.; Li, Y.; Qu, J.; Liu, Q.; Shen, Z.F.; Chen, X.G.; Yu, S.S. A new thiophene and two new monoterpenoids from Xanthium sibiricum. J. Asian Nat. Prod. Res. 2015, 17, 1039–1047.
- Zhang, L.; Chen, C.-J.; Chen, J.; Zhao, Q.-Q.; Li, Y.; Gao, K. Thiophene acetylenes and furanosesquiterpenes from Xanthopappus subacaulis and their antibacterial activities. Phytochemistry 2014, 106, 134–140.
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Esmat, A.; Mohamed, G.A. Thiotagetin B and tagetannins A and B, new acetylenic thiophene and digalloyl glucose derivatives from Tagetes minuta and evaluation of their in vitro antioxidative and anti-inflammatory activity. Fitoterapia 2018, 125, 78–88.
- Velíšek, J.; Cejpek, K. Biosynthesis of food constituents: Lipids. 1. Fatty acids and derived compounds—A review. Czech J. Food Sci. 2006, 24, 193–216.
- Field, J.A. Natural Production of Organohalide Compounds in the Environment. In Organohalide-Respiring Bacteria, 1st ed.; Adrian, L., Löffler, F.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 7–29.
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776.
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H. Anti-Inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders—A Review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58.
- Fizeșan, I.; Rusu, M.E.; Georgiu, C.; Pop, A.; Ștefan, M.G.; Muntean, D.M.; Mirel, S.; Vostinaru, O.; Kiss, B.; Popa, D.S. Antitussive, antioxidant, and anti-inflammatory effects of a walnut (Juglans regia L.) septum extract rich in bioactive compounds. Antioxidants 2021, 10, 119.
- Ranaweera, S.S.; Dissanayake, C.Y.; Natraj, P.; Lee, Y.J.; Han, C.H. Anti-inflammatory effect of sulforaphane on LPS-stimulated RAW 264.7 cells and ob/ob mice. J. Vet. Sci. 2020, 21, e91.
- Zhou, X.D.; Zhang, C.; He, S.; Zheng, B.; Zeng, K.W.; Zhao, M.B.; Jiang, Y.; Tu, P.F. New terpenoids and thiophene derivatives from the aerial parts of Artemisia sieversiana. Bioorg. Med. Chem. Lett. 2017, 27, 5441–5445.
- Ruan, J.; Li, Z.; Yan, J.; Huang, P.; Yu, H.; Han, L.; Zhang, Y.; Wang, T. Bioactive constituents from the aerial parts of Pluchea indica Less. Molecules 2018, 23, 2104.
- Chang, F.P.; Chen, C.C.; Huang, H.C.; Wang, S.Y.; Chen, J.J.; Yang, C.S.; Ou, C.Y.; Wu, J.B.; Huang, G.J.; Kuo, Y.H. A new bithiophene from the root of Echinops grijsii. Nat. Prod. Commun. 2015, 10, 2147–2149.
- Zhang, C.; Liu, B.Y.; Zeng, K.W.; Guo, X.Y.; Jiang, Y.; Tu, P.F. New sesquiterpene and thiophene derivatives from Artemisia rupestris. J. Asian Nat. Prod. Res. 2015, 17, 1129–1136.
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Tagetnoic acid, a new lipoxygenase inhibitor peroxy fatty acid from Tagetes minuta growing in Saudi Arabia. Nat. Prod. Res. 2020, 34, 474–481.
- Ibrahim, S.R.M.; Mohamed, G.A. Thiotagetin A, new cytotoxic thiophene from Tagetes minuta. Nat. Prod. Res. 2017, 31, 543–547.
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 25 December 2021).
- Chitsazian-Yazdi, M.; Agnolet, S.; Lorenz, S.; Schneider, B.; Es’haghi, Z.; Kasaian, J.; Khameneh, B.; Iranshahi, M. Foetithiophenes C-F, thiophene derivatives from the roots of Ferula foetida. Pharm. Biol. 2015, 53, 710–714.
- Sandjo, L.P.; Kuete, V.; Siwe, X.N.; Poumale, H.M.P.; Efferth, T. Cytotoxicity of an unprecedented brominated oleanolide and a new furoceramide from the Cameroonian spice, Echinops giganteus. Nat. Prod. Res. 2016, 30, 2529–2537.
- Boonruang, S.; Prakobsri, K.; Pouyfung, P.; Srisook, E.; Prasopthum, A.; Rongnoparut, P.; Sarapusit, S. Inhibition of human cytochromes P450 2A6 and 2A13 by flavonoids, acetylenic thiophenes and sesquiterpene lactones from Pluchea indica and Vernonia cinerea. J. Enzyme Inhib. Med. Chem. 2017, 32, 1136–1142.
- Xu, D.G.; Lv, W.; Dai, C.Y.; Zhu, F.F.; Xu, G.H.; Ma, Z.J.; Chen, Z. 2-(Pro-1-ynyl)-5-(5,6-dihydroxypenta-1,3-diynyl) thiophene induces apoptosis through reactive oxygen species-mediated JNK activation in human colon cancer SW620 cells. Anat. Rec. 2015, 298, 376–385.
- Kim, H.Y.; Kim, H.M.; Ryu, B.; Lee, J.S.; Choi, J.H.; Jang, D.S. Constituents of the aerial parts of Eclipta prostrata and their cytotoxicity on human ovarian cancer cells in vitro. Arch. Pharm. Res. 2015, 38, 1963–1969.
- Lee, J.S.; Ahn, J.H.; Cho, Y.J.; Kim, H.Y.; Yang, Y.I.; Lee, K.T.; Jang, D.S.; Choi, J.H. α-Terthienylmethanol, isolated from Eclipta prostrata, induces apoptosis by generating reactive oxygen species via NADPH oxidase in human endometrial cancer cells. J Ethnopharmacol. 2015, 169, 426–434.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318.
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64.
- Bhattacharjee, K.; Palepu, N.R.; Rao, K.M.; Joshi, S.R. Precursor-directed combinatorial biosynthesis of cephalosporin analogue by endolithic actinobacterium Streptomyces sp. AL51 by utilizing thiophene derivative. 3 Biotech 2018, 8, 31.
- Mbaveng, A.T.; Sandjo, L.P.; Tankeo, S.B.; Ndifor, A.R.; Pantaleon, A.; Nagdjui, B.T.; Kuete, V. Antibacterial activity of nineteen selected natural products against multi-drug resistant Gram-negative phenotypes. Springerplus 2015, 4, 823.
- Postigo, A.; Funes, M.; Petenatti, E.; Bottai, H.; Pacciaroni, A.; Sortino, M. Antifungal photosensitive activity of Porophyllum obscurum (Spreng.) DC.: Correlation of the chemical composition of the hexane extract with the bioactivity. Photodiagn. Photodyn. Ther. 2017, 20, 263–272.
- Postigo, A.; Schiavi, P.C.; Funes, M.; Sortino, M. Mechanistic studies of Candida albicans photodynamic inactivation with Porophyllum obscurum hexanic extract and its isolated thiophenic compounds. Photodiagn. Photodyn. Ther. 2019, 26, 420–429.
- Li, L.B.; Xiao, G.D.; Xiang, W.; Yang, X.; Cao, K.X.; Huang, R.S. Novel substituted thiophenes and sulf-polyacetylene ester from Echinops ritro L. Molecules 2019, 24, 805.
- Yu, S.J.; Zhang, J.S.; He, H.; Yu, J.H.; Bao, J.; Zhang, H. Thiophene enantiomers from the aerial parts of Eclipta prostrata. J. Asian Nat. Prod. Res. 2021, 23, 745–753.
- Cao, D.T.; Tran, V.H.; Vu, V.N.; Mai, H.D.T.; Le, T.H.M.; Vu, T.Q.; Nguyen, H.H.; Chau, V.M.; Pham, V.C. Antimicrobial metabolites from a marine-derived actinomycete Streptomyces sp. G278. Nat. Prod. Res. 2019, 33, 3223–3230.
- Gay, F.; Zougbédé, S.; N’dilimabaka, N.; Rebollo, A.; Mazier, D.; Moreno, A. Cerebral malaria: What is known and what is on research. Rev. Neurol. 2012, 68, 239–256.
- World Health Organization (WHO). World Malaria Report. 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 25 December 2021).
- Ibrahim, S.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F. Potential anti-malarial agents from endophytic fungi: A Review. Mini-Rev. Med. Chem. 2018, 18, 1110–1132.
- Bitew, H.; Mammo, W.; Hymete, A.; Yeshak, M.Y. Antimalarial activity of acetylenic thiophenes from Echinops hoehnelii Schweinf. Molecules 2017, 22, 1965.
- Zhao, M.P.; Liu, Q.Z.; Liu, Q.; Liu, Z.L. Identification of larvicidal constituents of the essential oil of Echinops grijsii roots against the three species of mosquitoes. Molecules 2017, 22, 205.
- Bi, Y.; Gao, C.; Yu, Z. Rhabdopeptides from Xenorhabdus budapestensis SN84 and their nematicidal activities against Meloidogyne incognita. J. Agric. Food Chem. 2018, 66, 3833–3839.
- Chen, J.; Chen, Y.; Gan, X.; Song, B.; Hu, D.; Song, B. Synthesis, nematicidal evaluation, and 3D-QSAR analysis of novel 1,3,4-oxadiazole-cinnamic acid hybrids. J. Agric. Food Chem. 2018, 66, 9616–9623.
- Tocco, G.; Eloh, K.; Onnis, V.; Sasanelli, N.; Caboni, P. Haloacetophenones as newly potent nematicides against Meloidogyne incognita. Ind. Crops Prod. 2017, 110, 94–102.
- Politi, F.A.S.; Bueno, R.V.; Zeoly, L.A.; Fantatto, R.R.; Eloy, J.O.; Chorilli, M.; Coelho, F.; Guido, R.V.C.; Chagas, A.C.S.; Furlan, M. Anthelmintic activity of a nanoformulation based on thiophenes identified in Tagetes patula L. (Asteraceae) against the small ruminant nematode Haemonchus contortus. Acta Trop. 2021, 219, 105920.
This entry is offline, you can click here to edit this entry!