Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Environmental Sciences
Pyrethrins and pyrethroids are a dominating group of insecticidal compounds that have been used for a long time and are still being used today, due to their potency and their variability. From natural pyrethrins, which can be utilized especially for their biodegradable properties, to the synthetic derivatives, pyrethroids, which may be used for their potency, this class of organic insecticides displays a lot of variability. It must be acknowledged that without plants, and plant metabolites, a great area of the insecticide compound class would be missing.
- pyrethrins
- pyrethroids
- natural compounds
1. Introduction
Botanical derivatives have served as fundamental pesticides for centuries and are renowned for their ecological properties [1,2]. Throughout history, various compounds such as sulfur, arsenic, and nicotine sulfate have been used to control pest infestations. After the Second World War, organochlorines and organophosphates emerged, marking a milestone in the development of pesticides. However, due to growing concerns about their environmental impact, alternative approaches such as integrated pest management (IPM) gained ground [3].
The complete ban on persistent organic pollutants, including DDT (dichlorodiphenyltrichloroethane), highlighted the need to re-evaluate pesticide use [4]. Subsequently, the advent of pyrethroids in the 1970s offered a promising alternative, offering efficacy and reduced environmental damage compared to their predecessors [5,6]. Despite their indisputable benefits in limiting agricultural losses and ensuring food security, the widespread use of pyrethroids and other pesticides has raised concerns about their negative effects on human health and the environment [7,8].
2. Pyrethrins
Plant materials containing pyrethrins have been traded in Europe since the middle of the 18th century. The species Chrysanthemum cinerariaefolium first appeared in the Dalmatia region of the Balkans and replaced a species originating from Persia, which proves that the true origin for the use of this plant is the Middle East, and dates to the 17th century. The first studies that set the foundation for the discovery of pyrethrins were elaborated in the 1920s, with the discovery of pyrethrins I and II [16]. These breakthroughs acted as a catalyst for the creation of a new market to sell the herb. The First World War also greatly influenced the countries that had a monopoly on the market, with Kenya becoming the leading exporter of plant products due to its higher concentration of active ingredients. Due to the pressures of World War II and other factors such as insect-borne diseases, these valuable pyrethrins were formulated as aerosols for their use against mosquitoes to decrease the transmission rate of malaria or yellow fever [16].
Another use of this class of plant compounds has been in agriculture as insecticides that are not highly toxic to mammals and are biodegradable. This led to their use on an industrial scale and the need to manufacture large quantities. In the production of pyrethrin-based insecticides, the starting point is generally the plant material, namely the mature inflorescences, which are dried and ground to a fine powder [2]. The powder can be used as such but can also be subjected to extraction with organic solvents.
Subsequently, different synthetic methods were developed for the 6 natural compounds, and new compounds structurally like the natural pyrethrins were created, thus generating a new class of compounds known as pyrethroids. These pyrethroids are superior to the natural compound due to their photostability, pyrethrins have a reduced photostability and are more biodegradable [17].
The term pyrethrum refers to the powdered and dried plant product extracted with organic solvents. In general, the term is used for the name of insecticide, either plant product or extract, which contains all the pyrethrins of this class of compounds. Several species are documented of which, after processing, the final product is called pyrethrum. The most famous species is C. cinerariaefolium (Dalmatian pyrethrum) due to its highest concentration of pyrethrins among all known species that produce this class of compounds, followed by C. coccineum (Persian pyrethrum) whose concentration in active principles is not as renowned [18], but their presence has been documented. Another species of the genus containing this class of compounds is C. balsamita also known as costmary [19]. Two other species from different genera that have been documented to contain pyrethrins are Anacyclus pyrethrum [20] and Tagetes erecta [21], all of which are members of the Asteraceae family.
2.1. Chrysanthemum cinerariaefolium
Chrysanthemum cinerariaefolium alias Tanacetum cinerariaefolium (Dalmatian pyrethrum) is the most industrially cultivated plant for pyrethrum production. The active ingredients are present in all organs of the plant but are concentrated in the inflorescences where, on the surface of the ovaries, there are secretory glandular hairs responsible for the abundant presence of pyrethrins in the plant tissues. Ref. [22] observed that the concentration of pyrethrins is higher in the epidermis of young leaves, and as the leaves mature the highest concentration of pyrethrins is found in the mesophyll of the leaf. This perennial species, since it is the most widely cultivated, has become the subject of most research papers based on the study of pyrethrins. From a morpho-anatomical point of view, the plant presents a capitulum-type inflorescence with central (tubular) yellow flowers and white radiating (ligulate) flowers, slender erect stems, and its leaves are alternate and pinnately lobed.
2.2. Chemical Structures and Classification
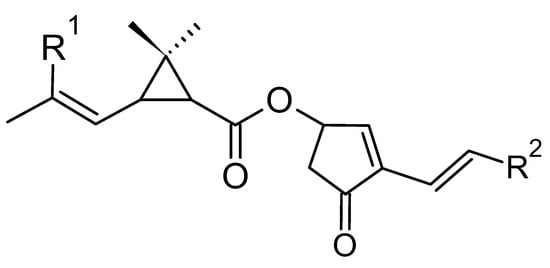
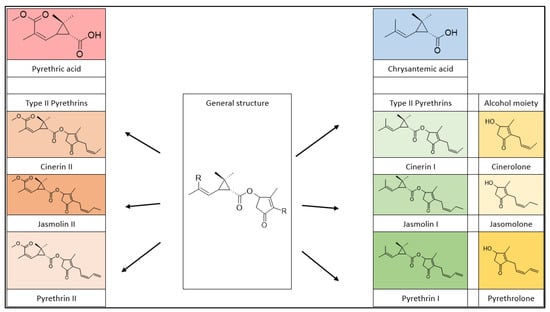
Natural pyrethrins are the six ester compounds that result from the condensation of one alcohol and acid, the general structure is shown below (Figure 1). The acid half may be pyrethric acid or chrysanthemic acid, and the alcohol half may be one of the unsaturated hydroxy-cycloketones (rethrolones): cinerolone, jasmolone, and pyrethrolone. If the compound has pyrethric acid in its structure, then the three acid-alcohol combinations belong to category I of pyrethrins, and if chrysanthemic acid is replaced by pyrethric acid then the three remaining compounds belong to category II of pyrethrins (Figure 2).

Figure 1. The general structure of pyrethrins.

Figure 2. Pyrethrin classification.
2.3. Biosynthesis
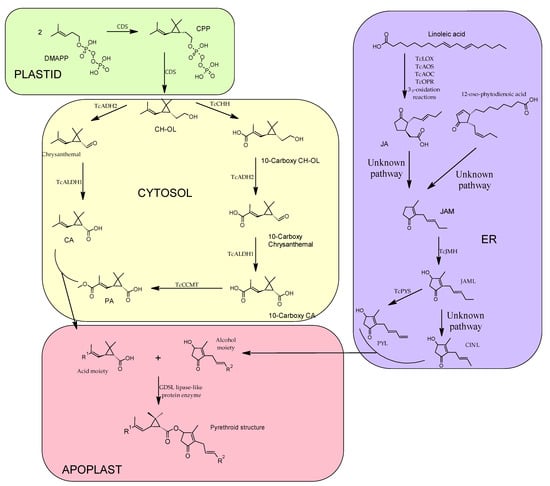
Pyrethrins are the final product of two major pathways merging, as shown below (Figure 3), one pathway is responsible for the synthesis of the acid moiety (crysanthemic acid—CA and pyrethric acid—PA) and another pathway is responsible for the synthesis of the alcohol moiety or rethrolones (jasmolone, cinerolone and pyrethrolone). It has been revealed that most of the reactions involved in the biosynthesis of pyrethrins, in seedlings of C. cinerariaefolium take place in plastids [28]; however, it is also stated that part of these reactions (the alcohol moieties biosynthesis) also take place in the ER (endoplasmic reticulum) and in the peroxisome [29].

Figure 3. General biosynthetic pathway of pyrethrins depicting the cellular domains where the different reactions of the biosynthesis take place. DMAPP—dimethylallyl diphosphate; CDS—chrysanthemyl diphosphate synthase; CPP—chrysanthemyl diphosphate; CH-OL—chrysanthemol; TcADH2—C. cinerariaefolium alcohol dehydrogenase 2; TcALDH1—C. cinerariaefolium aldehyde dehydrogenase 1; CA—crysanthemic acid; PA—pyrethric acid; TcCHH—chrysanthemol 10-hydroxylase; TcCCMT—10-carboxychrysanthemic acid 10-methyltransferase; TcLOX—T. cinerariaefolium lipoxygenase; TcAOS—allene oxidase synthase; TcAOC—allene oxide cyclase; TcOPR—cis (1)-12-oxo-phytodienenic acid reductase; JA—jasmonic acid; JAM—jasmone; TcJMH—jasmone hydroxylase; JAML—jasmolone; TcPYS—Pyrethrolone synthase; PYL—pyrethrolone; CINL—cinerolone.
In the synthesis of the acid moiety, the starting compound is dimethylallyl diphosphate (DMAPP), which is the universal terpene precursor. Two molecules of the DMAPP are condensed resulting in chrysanthemyl diphosphate (CPP) and after eliminating the phosphate group by hydrolysis the final product of this step is chrysanthemol (CH-OL). This two-step reaction is catalyzed by the enzyme chrysanthemyl diphosphate synthase (CDS) also known as chrysanthemol synthase (CHS) [30], CPP is, because it is an irregular terpene due to the head-to-middle dimerization of DMAPP. CH-OL is converted into CA by two-step oxidation, firstly the alcohol is transformed into an aldehyde (chrysanthemal), catalyzed by C. cinerariaefolium alcohol dehydrogenase 2 (TcADH2), followed by an oxidation to CA, a reaction catalyzed by and aldehyde dehydrogenase 1 (TcALDH1) [31].
2.4. Mechanism of Action
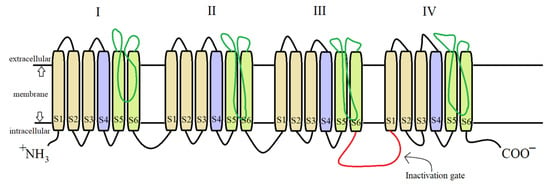
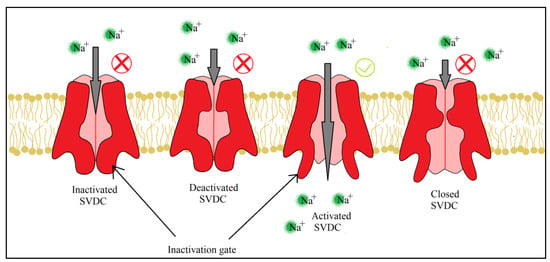
For both pyrethrins and pyrethroids the mechanism of action follows the same pattern, yet because of the biochemical engineering that led to the creation of pyrethroids, the synthetic class has a longer effect and a bigger potency. It has been determined, by molecular genetic studies done on houseflies centered on the resistance of pyrethroids [39], that the central site of insecticidal action revolves around the sodium voltage-dependent channels (SVDC). SVDC consists of a long transmembrane amino acid chain (Figure 4), with the amino and carboxy termini situated intracellular, forming two major subunits: α subunit (which is the main center of action) and aβ auxiliary subunit (responsible for the modulation of sodium channel gating and/or protein expression) [40]. The α subunit is composed of four main domains (I–IV), each domain has six transmembrane segments (S1–S2). The S4 contains positively charged amino acids and it acts as the voltage sensor when the membrane is depolarized receiving a confirmation charge that opens the channel. The channel is represented by the S5, S6, and the linkage segment between them, this segment also confers specificity to Na+ ions. There is an amino acid linkage chain between the domains III and IV which represents the inactivation gate. The channel can exist in four states due to the inactivation gate and one more site named the “activation gate”: the first state when the membrane potential is resting, the channel is closed but the inactivation gate is opened (closed state) when the depolarization is detected, the channel fully opens and the Na+ ions can freely pass thru (activated state). The inactivation state is initiated by the inactivation gate closing, clogging up the channel, after this step the channel finial closes and enters its deactivated state.

Figure 4. Detailed structure of the α subunit of the SVDC, the transmembrane amino acid chain with its four domains (I–IV), each of them having six transmembrane segments (S1–S6).
SVDC and potassium voltage-dependent channels (PVDC) are responsible for the movement of Na+ and K+ across the membrane which create action potentials, electrical impulses that travel along the neurons [41]. When a neurotransmitter binds to its receptor, in the synapse, a depolarization of the neuron membrane takes place and S4 segments of the SVDC detect the difference in charge, activating the channel and letting Na+ enter the neuron which leads to a further depolarizing of the membrane. This increasing concentration of Na+ oversees the rising phase of the action potential, leading to the membrane polarity switch. The following phase, the falling of the action potential, is produced by the inactivation and closing of the SVDC and the activation and opening of the PVDC, letting the K+ leave the neuron which leads to the hyperpolarization of the membrane. Finally, the PVDC closes and the membrane potential is back to its resting phase. All four stages of the ion channel can be viewed below (Figure 5).

Figure 5. Pyrethroids and their effects on ion channels, the four states of SVDC, are dependent on the inactivation and activation gate. Readaptation of the representation from [41].
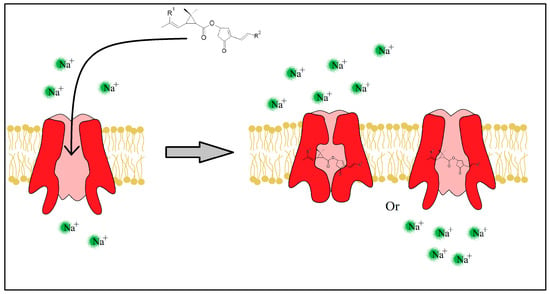
The pyrethrins and pyrethroids lessen the peak of Na+ ions (the rising phase) by slowing the SVDC activation state [42,43], but also prolong the SVDC opening time by slowing the activated and inactivated state of the channel (Figure 6). Immediately after the fast absorption of the insecticide molecule through the insect’s cuticle, the neurotransmission fails causing a paralyzing state and possibly death in seconds or minutes.

Figure 6. Insecticide molecule mechanism of action, by slowing the Inactivated or Activated state of the SVDC.
2.5. General and Pharmaceutical Uses
Since the newly developed pyrethroids have been on the market, the usage of pyrethrins in different forms for repelling insects has greatly decreased. Although the insecticidal power of pyrethrins and pyrethroids is recognized, they are generally associated with different insecticides such as seasmin, sulfoxide, and dicarboximide, for the synergetic potentiation of the effect [49]. Their primary use as a household insecticide is still noted today, different types of sprays, aerosols, solutions, and incense are used to prevent unwanted insects in the household.
A multitude of veterinary products containing this class of compounds are used for their properties to protect pets against insect pests, amongst these formulations can be found collars, powders, shampoos, baths, aerosols, sprays, etc. Among all these formulations for veterinary use, two categories of products can be distinguished, namely products using an organic solvent (alcohol or petroleum), which are more prone to toxic and/or adverse reactions, and products using a water-based solvent, whose bioavailability is relatively low.
3. Pyrethroids
3.1. General Considerations about Pyrethroids
Pyrethrin derivatives, known as pyrethroids, have a significant historical trajectory dating back to the mid-20th century, characterized by a series of crucial advancements in the realm of synthetic insecticide development. The milestone achievements include the synthesis of allethrin and bioallethrin in 1949, setting the stage for the subsequent introduction of the first-generation synthetic pyrethroid, resmethrin, in 1962, achieved through structural modifications of natural pyrethrins to enhance stability and elevate insecticidal efficacy [57,58].
The progression in this field expanded with the development of bioresmethrin in 1967, marking the commercial application of pyrethroids in the late 1960s, coinciding with the emergence of potent insecticides like cypermethrin and deltamethrin and solidifying the role of pyrethroids in the domain of pest management [59,60]. By 1983, the widespread utilization of pyrethroids encompassed over 33 million hectares annually, representing a significant share of the global insecticide market at 25.1% [3].
Categorized into type I and type II pyrethroids based on distinct chemical attributes influencing their insecticidal properties, these compounds have found extensive use across various sectors. Additionally, the incorporation of piperonyl butoxide as a synergist in commercial pyrethroid formulations has significantly contributed to their sustained efficacy in pest control [62,63].
Despite their pivotal role in pest control, concerns have surfaced regarding the impact of pyrethroids on non-target species and potential health implications for humans. Research efforts have focused on understanding specific toxicity biomarkers associated with pyrethroid exposure, shedding light on their adverse effects on various ecosystems [64,65,66,67,68].
3.2. Classification of Pyrethroids
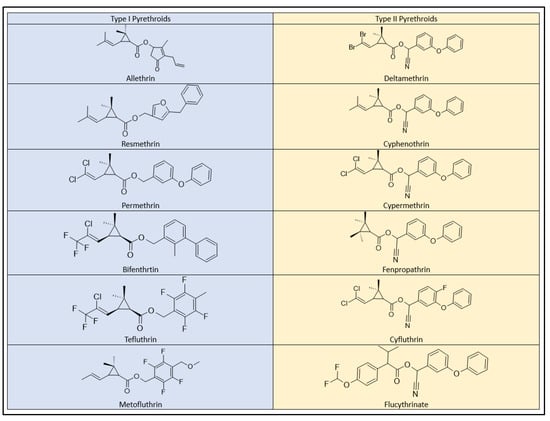
Pyrethroid pesticides exhibit a clear classification into two distinctive categories, known as Type I and Type II, based on their behavioral toxicity and the presence or absence of an α-cyano group within their molecular structures [73]. This division is also reflected in the acute toxicity assessments, where most pyrethroids are grouped into classes I and II (Figure 7), indicating their respective levels of toxicity in rodent models.

Figure 7. Type I and Type II pyrethroids structures.
Type I pyrethroids, which include allethrin, permethrin, resmethrin, bifenthrin, d-phenothrin, and tetramethrin, do not contain the α-cyano group in their chemical structure. As a result, they exhibit comparatively lower toxicity. In contrast, Type II pyrethroids, such as cypermethrin, deltamethrin, cyhalothrin (lambda), cyfluthrin, and fenvalerate (esfenvalerate), incorporate the α-cyano group, making them notably more toxic [58]. Type II pyrethroids have been associated with salivation, the choreoathetosis-salivation syndrome (CS), and motor dysfunction in mammals [71,72,73,74,75].
Human biomonitoring (HBM) studies typically monitor pyrethroid exposure through the detection of five specific metabolites in urine: 3-phenoxybenzoic acid (3-PBA), cis- and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (cis-DCCA and trans-DCCA), 4-fluoro-3-phenoxybenzoic acid (F-PBA), and 3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane carboxylic acid (DBCA). Among these, DBCA is specific to deltamethrin, and F-PBA is linked to cyfluthrin but can originate from both isomers. The metabolites are cis- and trans-DCCA are produced from the cis and trans isomers of permethrin, cypermethrin, and cyfluthrin, while 3-PBA is a common metabolite observed with various pyrethroids, including permethrin, cypermethrin, and deltamethrin [77].
3.3. Synthesis Strategies of Pyrethroids: Approaches and Mechanistic Considerations
The synthesis of pesticides encompasses various strategies, each tailored to facilitate the creation of novel compounds with specific biological activities. Among the prominent methods, biomimetic synthesis simulates natural reactions within living organisms [80], while substructure splicing amalgamates active pesticide fragments to generate compounds with enhanced biological efficacy [81]. For the efficient synthesis of pyrethroids, the primary approach involves esterification, necessitating the initial production of chrysanthemic acid derivatives and alcohols (or aldehydes) [82,83,84,85].
Pyrethroids, renowned for their potent insecticidal properties and low mammalian toxicity, have evolved significantly, leveraging structural modifications to enhance stability and effectiveness [86]. The integration of aromatic groups into the alcoholic moiety has notably contributed to the improved stability of pyrethroids, allowing for their widespread application in crop protection [82,83,84,85]. This stability feature has propelled the exploration of various modifications, particularly in the alcohol and acid segments, underscoring the versatility of the synthesis approach [82,83,84,85].
3.4. Toxicological Insights into Pyrethroids: Human and Environmental Implications
Pyrethroids, a class of pesticides, have gained recognition for their relatively low toxicity in humans compared to other pesticide groups, including organochlorines, organophosphates, and carbamates [5]. However, type II pyrethroids have demonstrated greater acute oral toxicity than their counterparts, warranting a detailed assessment of their effects [5]. The toxicity profile of pyrethroids is diverse, with type I pyrethroids causing reversible skin and eye irritation upon exposure, while type II pyrethroids pose more severe risks due to their neurotoxic nature, often leading to fatal outcomes [5].
The human metabolism of pyrethroids involves various enzymes, including cytochrome P450s and carboxylesterases, contributing to the degradation of these compounds [87]. Despite their lower impact on humans relative to insects, pyrethroids can still induce alterations in various physiological functions, emphasizing the need for a comprehensive understanding of their toxicological effects [61].
3.4.1. Toxicity in Humans
Pyrethroid poisoning primarily results from the disruption of sodium and chloride
channels. Type I pyrethroids cause distinct symptoms known as type I syndrome, while
type II pyrethroids, characterized by an additional cyan group in their chemical structure,
elicit type II syndrome [88]. Instances of pyrethroid-induced cardiotoxicity have been
reported, particularly associated with prallethrin, a common household pesticide used
against mosquitoes, cockroaches, and houseflies [89].
Exposure to prallethrin has been linked to alterations in plasma biochemical profiles,
with significant changes observed in glucose, phospholipids, nitrite, nitrate, and lipid
peroxidase levels [90]. Additionally, allethrin and prallethrin exposure have been associated
with increased MUC5AC expression in human airway cells and heightened reactive oxygen
species production, underlining their potential impact on respiratory health [91].
Moreover, prallethrin poisoning has been implicated in gastrointestinal, respiratory,
and nervous system disturbances, leading to metabolic acidosis and cardiac conduction
disturbances [92]. Accidental and suicidal ingestions are the primary causes of pyrethroid
poisoning in humans [93], with dermal exposure also being a common entry route [94–96].
Pyrethroid poisoning primarily results from the disruption of sodium and chloride
channels. Type I pyrethroids cause distinct symptoms known as type I syndrome, while
type II pyrethroids, characterized by an additional cyan group in their chemical structure,
elicit type II syndrome [88]. Instances of pyrethroid-induced cardiotoxicity have been
reported, particularly associated with prallethrin, a common household pesticide used
against mosquitoes, cockroaches, and houseflies [89].
Exposure to prallethrin has been linked to alterations in plasma biochemical profiles,
with significant changes observed in glucose, phospholipids, nitrite, nitrate, and lipid
peroxidase levels [90]. Additionally, allethrin and prallethrin exposure have been associated
with increased MUC5AC expression in human airway cells and heightened reactive oxygen
species production, underlining their potential impact on respiratory health [91].
Moreover, prallethrin poisoning has been implicated in gastrointestinal, respiratory,
and nervous system disturbances, leading to metabolic acidosis and cardiac conduction
disturbances [92]. Accidental and suicidal ingestions are the primary causes of pyrethroid
poisoning in humans [93], with dermal exposure also being a common entry route [94–96].
3.4.2. Biological Mechanisms and Environmental Impact
In both humans and animals, the nervous system serves as the primary target of
pyrethroids, leading to acute neurobehavioral effects. The classification of pyrethroids into
type I and type II groups is based on their specific neurotoxic manifestations in rodents and
other species [109–111]. Environmental exposure to pyrethroids can be particularly harmful
to aquatic life, emphasizing the need for stringent precautionary measures [112–114].
Similarly, pyrethrin and pyrethroid products can pose risks to avian species, particularly in
the presence of certain carriers or propellants in spray formulations [115].
4. Conclusions
Pyrethrins and pyrethroids are a dominating group of insecticidal compounds that
have been used for a long time and are still being used today, due to their potency and their
variability. From natural pyrethrins, which can be utilized especially for their biodegradable
properties, to the synthetic derivatives, pyrethroids, which may be used for their potency,
this class of organic insecticides displays a lot of variability. It must be acknowledged that
without plants, and plant metabolites, a great area of the insecticide compound class would
be missing.
In both humans and animals, the nervous system serves as the primary target of
pyrethroids, leading to acute neurobehavioral effects. The classification of pyrethroids into
type I and type II groups is based on their specific neurotoxic manifestations in rodents and
other species [109–111]. Environmental exposure to pyrethroids can be particularly harmful
to aquatic life, emphasizing the need for stringent precautionary measures [112–114].
Similarly, pyrethrin and pyrethroid products can pose risks to avian species, particularly in
the presence of certain carriers or propellants in spray formulations [115].
4. Conclusions
Pyrethrins and pyrethroids are a dominating group of insecticidal compounds that
have been used for a long time and are still being used today, due to their potency and their
variability. From natural pyrethrins, which can be utilized especially for their biodegradable
properties, to the synthetic derivatives, pyrethroids, which may be used for their potency,
this class of organic insecticides displays a lot of variability. It must be acknowledged that
without plants, and plant metabolites, a great area of the insecticide compound class would
be missing.
However, from the pyrethroid’s point of view, the constant demand for a new molecule
that is less toxic, and more biodegradable, yet its potency does not lessen, may be an important
catalyst to chemical engineering a compound that may satisfy all the necessities. The
impetus of constantly developing and innovating the field of pyrethrins and pyrethroids
also urges the research of the environmental impact of these compounds and the toxic
effects on humans and animals, which in some cases may be fatal or threatening.
To conclude, this promising potential of pyrethrins and pyrethroids seems likely to
persist in the future and needs constant innovation since all areas in which these insecticides
are used, from agriculture, household insecticides, veterinary industry to the pharmaceutical
and medical industry, require the development of new molecules or methods to analyze
these compounds for different purposes.
From the natural compound’s standpoint, the fully biosynthetic pathway of pyrethrins
has yet to be elucidated, nonetheless clarifying the full path may be a key insight into
genetically ingenerating subspecies of plants that may yield more pyrethrins, helping the
pyrethrum industry flourish.
that is less toxic, and more biodegradable, yet its potency does not lessen, may be an important
catalyst to chemical engineering a compound that may satisfy all the necessities. The
impetus of constantly developing and innovating the field of pyrethrins and pyrethroids
also urges the research of the environmental impact of these compounds and the toxic
effects on humans and animals, which in some cases may be fatal or threatening.
To conclude, this promising potential of pyrethrins and pyrethroids seems likely to
persist in the future and needs constant innovation since all areas in which these insecticides
are used, from agriculture, household insecticides, veterinary industry to the pharmaceutical
and medical industry, require the development of new molecules or methods to analyze
these compounds for different purposes.
From the natural compound’s standpoint, the fully biosynthetic pathway of pyrethrins
has yet to be elucidated, nonetheless clarifying the full path may be a key insight into
genetically ingenerating subspecies of plants that may yield more pyrethrins, helping the
pyrethrum industry flourish.
This entry is adapted from the peer-reviewed paper 10.3390/plants12234022
This entry is offline, you can click here to edit this entry!
