Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Drug-Induced Sleep Endoscopy (DISE) enables the three-dimensional and dynamic visualization of the upper airway (UA) during sleep, which is useful in selecting the best treatment option for obstructive sleep apnea (OSA) patients, particularly for surgical procedures.
- obstructive sleep apnea
- drug-induced sleep endoscopy
- upper airways surgery
1. Introduction
Obstructive sleep apnea (OSA) is the most common and under-diagnosed sleep-disordered breathing (SDB) disease, with a high prevalence in the adult population, which is reported from 13% to 33% in men and 6% to 19% in women, considering the methodological heterogeneity of the epidemiological studies [1]. The pathophysiology of OSA is quite complex and is related to specific pathophysiological traits: high upper airway (UA) collapsibility, UA muscular impairment, unstable ventilatory control system or loop gain (LG), and low arousal threshold (AT). Even though the pharyngeal collapsibility (anatomical factor) is the most important factor amongst the PTs, in more than 70% of OSA patients, it could be a relative contribution or combination of each of the anatomical and non-anatomical PTs [2].
Pharyngeal collapsibility is measured by the passive critical closing pressure (Pcrit), where a high Pcrit (>2 cm H2O) is related to high pharyngeal collapsibility [2]. Continuous Positive Airway Pressure (CPAP) represents the first-line treatment option for OSA, but with a long-term decrease in compliance from 50 to 70%. Consequently, alternative treatment options are being advocated, such as UA surgery, mandibular advancement devices (MAD), and positional therapy [2][3].
The main result achievable with surgical treatment is the remodeling of UA, with the expansion and/or stabilization and/or tissue removal to different UA levels, which determine the improvement of UA resistance to collapse during obstructive sleep events. In this view, a targeted approach based on medical history, sleep studies, and clinical examination is of pivotal importance for the detection of the presence and preponderance of anatomical factors in UA collapsibility and for the selection of the best surgical procedures for each OSA patient [4].
Drug-induced Sleep Endoscopy (DISE) represents one of the most widespread diagnostic procedures for the identification of UA anatomical sites of obstruction in OSA patients, classifying the findings according to the severity and configuration of UA collapse [1]. DISE methodology, feasibility, and reliability have been extensively investigated since its first introduction by Pringle and Croft in 1991, as demonstrated by the widely published literature data [1][5][6]. There is general agreement that DISE enables analysis of the non-REM N2 sleep stage and can provide important dynamic and three-dimensional views of the main sites of UA collapse during apnea events [7].
2. Pharyngeal Patterns of Collapse in Obstructive Sleep Apnea
2.1. DISE and UA Pattern of Collapse/Obstruction (Table 1)
During DISE, it is possible to visualize different patterns and grades of UA collapse and obstruction at different levels and areas of the UA: at the soft palate, at the oropharyngeal lateral walls, at the base of the tongue, at the epiglottis, at the oral tongue, and at the nasal cavities. The patterns might be summarized as follows:
Table 1. DISE descriptive classification.
| UA Site | UA Pattern of Collapse | |
|---|---|---|
| Patterns of Soft Palate Collapse | concentric collapse |
|
| antero-posterior collapse |
|
|
| Patterns of Oropharyngeal Lateral Walls | latero-lateral collapse |
|
| Patterns of Base of Tongue Collapse | antero-posterior collapse |
|
| Patterns of Epiglottic Collapse | antero-posterior collapse latero-lateral collapse |
|
2.1.1. Patterns of Soft Palate Collapse
Concentric Pattern
One of the most clinically significant patterns of collapse visualized at the soft palate during DISE is defined as concentric (Figure 1), and it is referred to as the primary complete concentric collapse (CCC) DISE pattern. Otherwise, Van de Perck et al. [8] reported that in some OSA patients, careful observation of the CCC pattern during DISE could detect a simultaneous latero-lateral and antero-posterior collapse (LL-AP) at the velum level (Figure 2).
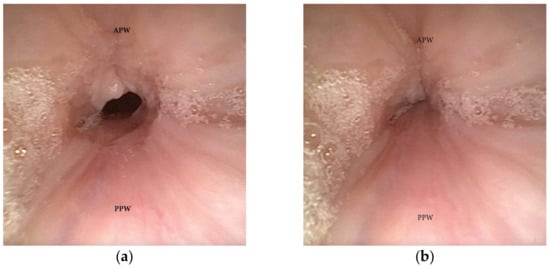
Figure 1. (a,b) DISE pattern of complete concentric collapse (CCC) of the soft palate. (APW: anterior pharyngeal wall; PPW: posterior pharyngeal wall).
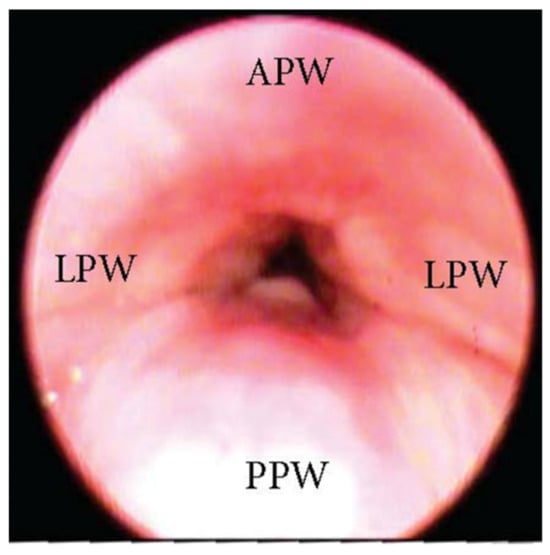
Figure 2. DISE pattern of latero-lateral/antero-posterior collapse of the soft palate. (APW: anterior pharyngeal wall; PPW: posterior pharyngeal wall; LPW: lateral pharyngeal wall).
Antero-Posterior (AP) Collapse
An AP collapse at the soft palate level during DISE can be defined as primary if it is due to an intrinsic collapse modality of the soft palate (Figure 3). However, in a significant percentage of OSA patients, a soft palate AP collapse can be driven by the posterior displacement of the junctional tongue (Figure 4). Furthermore, in the case of primary AP palatal collapse, the analysis of the soft/hard palate angle is also of pivotal importance, as a primary AP pattern of collapse is usually associated with an acute angle in which the posterior pharyngeal wall is a short distance from the soft palate [9].
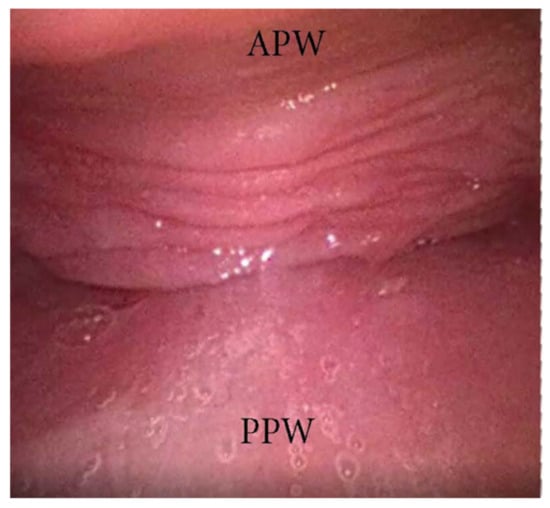
Figure 3. DISE pattern of primary antero-posterior collapse of soft palate. (APW: anterior pharyngeal wall; PPW: posterior pharyngeal wall).
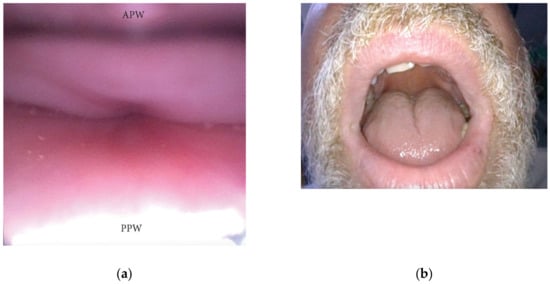
Figure 4. (a,b) DISE pattern of secondary antero-posterior collapse of the soft palate due to the posterior displacement of the junctional tongue (APW: anterior pharyngeal wall; PPW: posterior pharyngeal wall).
2.1.2. Patterns of Oropharyngeal Lateral Walls
The oropharyngeal wall collapse frequently assumes a latero-lateral pattern during DISE, which is often associated with a more severe pharyngeal collapsibility (Figure 5). Otherwise, in adult OSA patients, the presence of palatine tonsil hypertrophy also results in partial or complete latero-lateral occlusion of the oropharynx during apnea events (Figure 6). Furthermore, in most OSA patients, a latero-lateral pharyngeal collapse may merge both palatine tonsil hypertrophy and a more severe extrinsic oropharyngeal lateral wall collapse.
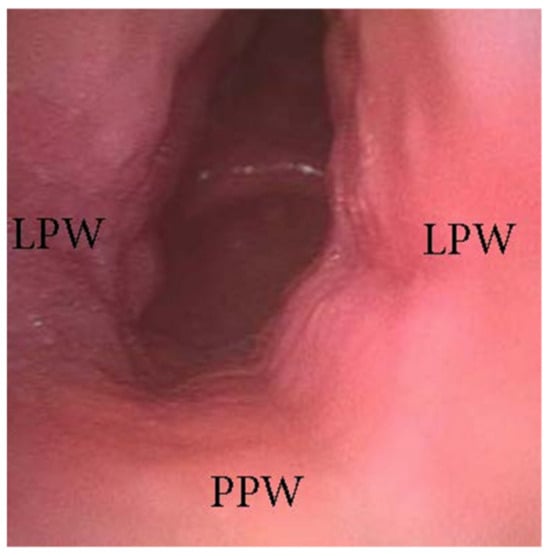
Figure 5. DISE pattern of latero-lateral collapse of the lateral oropharyngeal wall (PPW: posterior pharyngeal wall; LPW: lateral pharyngeal walls).
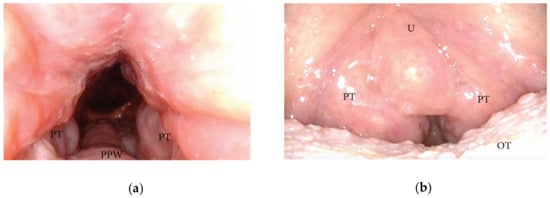
Figure 6. (a,b) DISE pattern of lateral oropharyngeal wall obstruction due to tonsillar hypertrophy (PPW: posterior pharyngeal wall; PT: palatine tonsils; U: uvula; OT: oral tongue).
2.1.3. Patterns of the Base of Tongue Collapse
The collapse at the level of the base of the tongue can be related to a higher degree of lingual tonsil hypertrophy (Figure 7) or an antero-posterior collapse of a vertically positioned muscular tongue base (Figure 8).
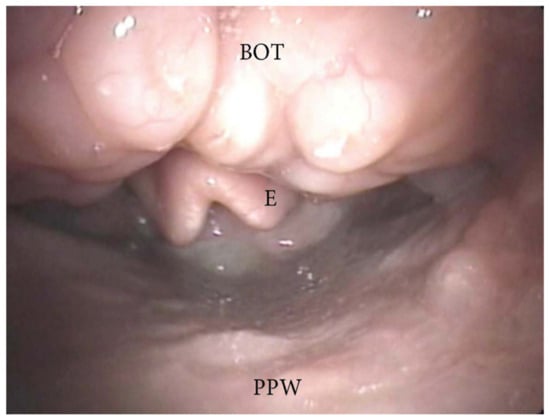
Figure 7. DISE pattern of obstruction due to lingual tonsil hypertrophy (BOT: base of the tongue; PPW: posterior pharyngeal wall; E: epiglottis).
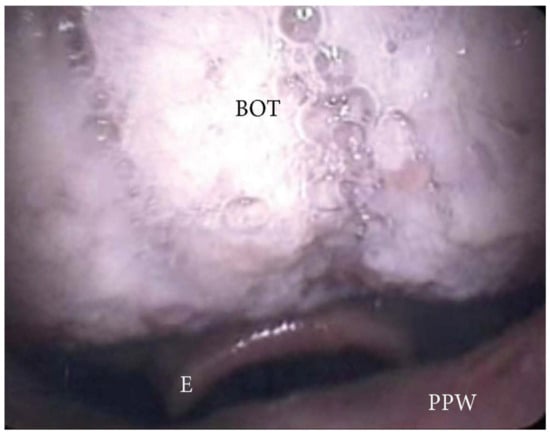
Figure 8. DISE pattern of vertically positioned muscular base of the tongue (BOT: base of the tongue; PPW: posterior pharyngeal wall; E: epiglottis).
2.1.4. Patterns of Epiglottic Collapse
During DISE, it is possible to analyze a primary anteroposterior configuration of the collapse of the epiglottis, also known as the “trapdoor” phenomenon (Figure 9). More frequently, different secondary collapses are observed due to hypopharyngeal latero-lateral occlusion (Figure 10) or obstruction caused by lymphatic tissue hypertrophy at the base of the tongue (Figure 7). Additionally, an aryepiglottic fold pattern of collapse can be observed, even in the adult OSA population, similar to laryngomalacia in the pediatric population (Figure 11).
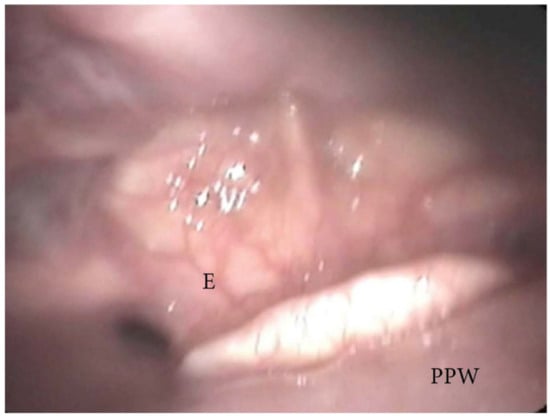
Figure 9. DISE pattern of epiglottic trapdoor collapse (PPW: posterior pharyngeal wall; E: epiglottis).
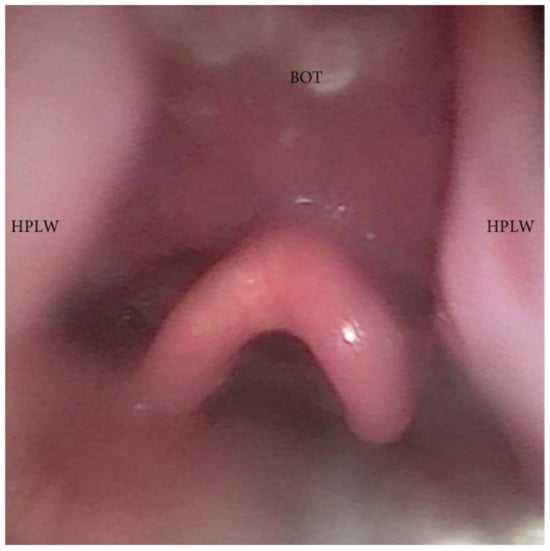
Figure 10. DISE pattern of hypopharyngeal latero-lateral collapse (HPLW: hypopharyngeal lateral wall; BOT: base of the tongue).
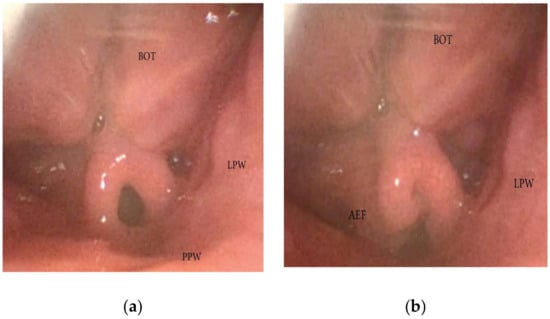
Figure 11. (a,b) DISE pattern of aryepiglottic collapse (BOT: base of the tongue; PPW: posterior pharyngeal wall: AEF: aryepiglottic fold; LPW: lateral pharyngeal wall).
2.1.5. Oral Tongue Patterns of Collapse
During DISE, the collapse of the oral tongue should also be analyzed through the mouth. In a significant percentage of OSA patients, a relative disproportion between the oral cavity and the tongue volume can be visualized, leading to posterior displacement of the junctional tongue and a partial or complete obstruction of the soft palate. This finding is suggestive of a tongue-driven anteroposterior soft palate collapse during DISE (Figure 4).
2.1.6. Nasal Obstruction and Pattern of Collapse
The nasal cavity is usually described as the entrance (rigid portion) of the Starling resistor model, which explains the differential pressure between nasal input pressure, pharyngeal surrounding pressure, and laryngotracheal output pressure [10]. While the nose can be considered the rigid segment in this model, there may be a different degree of collapse at the level of the nasal valve area. Additionally, the presence of septal nasal deviation, inferior turbinate hypertrophy, sino-nasal polyposis, or concha bullosa can cause significant nasal obstruction, leading to an increase in a varying degree of negative intraluminal pharyngeal pressure.
The complexity of OSA pathophysiology consists of different endotypes, which need to be identified to achieve an effective multimodal therapeutic approach. Anatomical factors predisposing to UA obstruction during sleep represent the most important, but not the single OSA pathophysiological factor. Eckert et al. found that more than 70% of OSA patients’ non-anatomical factors (high LG, low AT, and neuromuscular impairment) could contribute to the partial or complete UA occlusion [11].
A hyper-responsive ventilation control (high LG) determines a willingness to the ventilatory overshoot response for a minimal CO2 perturbation during sleep, resulting in excessive removal of CO2 and triggering a situation of cyclical apneic events. A low AT determines respiratory overshoots, which increases ventilator instability control and pharyngeal muscular instability and leads to the recurrence of the obstructive events. Moreover, a low AT could cause sleep fragmentation, contributing to daytime pathologic sleepiness.
Finally, several mechanisms of damage to UA afferent and efferent nerves have been identified and can cause a reduction in the neuromuscular compensation activity of UA muscles during sleep [2][11].
Therefore, because of those limitations, it has been recommended to leave enough room for additional descriptions of specific DISE findings in the final report.
-
Velum and Oropharyngeal patterns of collapse:
The distinction between CCC and LL-AP collapse in the velum region is crucial. This is because pure concentric collapse is a contraindication for hypoglossal nerve stimulation [12][13], whereas the LL-AP could take into consideration hypoglossal nerve stimulation as a treatment option. Similarly, differentiating primary vs. secondary tongue-driven antero-posterior palate collapse can significantly impact the outcomes of non-CPAP treatment options. Primary palatal collapse may require surgical therapy addressing the soft tissue or bone dimension (pharyngoplasty techniques), while secondary collapse may benefit from mandibular advancement devices or hypoglossal nerve stimulation. Furthermore, analyzing the soft/hard palate angle is critical in cases of primary AP palatal collapse. An acute angle often correlates with a short distance between the soft palate and posterior pharyngeal wall, making pharyngeal surgical techniques focused on palate-pharyngeal soft tissue remodeling contraindicated due to their low percentage of positive outcomes [9][14]. Furthermore, DISE can include the observation of the salpingopharyngeal fold, suggesting an increased UA collapsibility associated with negative effort dependence and lateral wall collapse, which may change the surgical planning and outcomes for OSA treatment [15].
-
Tongue patterns of collapse:
The distinction between a tongue base obstruction due to different grades of lymphatic tissue hypertrophy, with or without secondary epiglottic collapse, and a tongue base collapse of a vertically positioned muscular base of the tongue guides the surgical approach through tongue base reduction (transoral robotic or coblation techniques) in the first case or the use of a mandibular advancement device, skeletal surgery, or hypoglossal nerve stimulation in the second case [7][16][17].
-
Epiglottis patterns of collapse:
During DISE, it is possible to observe different configurations of epiglottis collapse, including the primary anteroposterior configuration known as the “trap door” phenomenon, as well as secondary collapse due to hypopharyngeal latero-lateral occlusion or anterior-posterior collapse of the lymphatic lingual tonsil hypertrophy. Furthermore, the length of the glosso-epiglottic fold may determine the type of collapse noted, as would the bulk of the most posterior aspect of the tongue base. If there is a long glosso-epiglottic fold and therefore a lot more space between the base of the tongue and the epiglottis, then one would more likely encounter the trap-door phenomenon. Conversely, if the valecullae are extremely narrow with a short gloss-epiglottic fold and a shorter but tighter glosso-epiglottic fold, then the pattern of collapse would be different [18]. Additionally, an ary-epiglottis fold pattern of collapse may also be observed, which is similar to laryngomalacia in pediatric populations. Different approaches may be necessary to address each type of epiglottis collapse, and a careful selection of the therapy is mandatory based on the previously described causal mechanism (epiglottoplasty techniques, partial epiglottectomy). It is also important to note that arytenoid edema, often caused by esophageal-laryngeal reflux, may be a contributing factor in a laryngeal collapse during apnea events [19].
3. Conclusions
OSA treatment overall strategy is steadily moving from a CPAP-centered “one-size-fits-all” approach to tailored multimodality treatment. Patients with high UA collapsibility are the best candidates for CPAP, with an adjunctive or salvage role for surgical treatment and oral appliances. Otherwise, OSA patients with mild-to-moderate UA collapsibility may be a good candidate for UA surgical treatment. However, more than 70% of OSA patients also have concomitant altered anatomical factors and non-anatomical factors (LG, AT, and neuromuscular impairment), with different impacts in each patient. Therefore, these patients may benefit from anatomical or non-anatomical treatments, such as UA surgery, MAD, and hypoglossal nerve stimulation.
DISE enables the three-dimensional and dynamic visualization of the UA during sleep, which is useful in selecting the best treatment option for OSA patients, particularly for surgical procedures. The assessment of the UA in the awake and asleep states can significantly differ in all the main pharyngeal levels involved in hypopnea or apnea events. A considerable amount of literature focuses on comparing DISE with natural sleep, interobserver reliability, decision-making processes for treatment options, and its predictive impact on surgical outcomes.
Finally, OSA patients could undergo DISE with airflow monitoring and nasal positive airway pressure titration. Consequently, it is possible to determine the visual and airflow-based levels of pharyngeal opening pressure (PhOP). The DISE-PhOP evaluation can be performed visually or on an integrated recording platform to acquire endoscopic images, flow, and effort (belts or catheters) from the patient’s UA. A detailed description of the DISE-PhOP setup has been previously published [20]. In short, the nasolaryngoscope was passed through a custom-fitted mask into the nasal cavity. PAP (S9 VPAP, ResMed Inc., San Diego, CA, USA) was applied and titrated using a nasal mask (Pulmodyne, Indianapolis, IN, USA). Propofol anesthesia was administered to achieve sedation with a target Bispectral Index (BIS) of 50–70. Once a stable sedation plan is achieved, PAP titration starts. PhOP is derived from DISE-PAP visually by the elimination of inspiratory effort or from pressure-flow relationships, as previously described [20]. PAP stars at 4 cm H2O after respiratory events were observed at atmospheric pressure, with further titration at increments of 1 cm H2O at the termination of obstructive apnea or hypopnea. The evidence so far is that patients with a PhOP of less than 8 have a better response to hypoglossal nerve stimulation therapy, suggesting a tongue-driven mechanism. Preliminary data suggests that higher values of PhOP respond better to expansion pharyngoplasty and Maxillo-mandibular advancement. Visual DISE-PhOP could represent a method to measure UA collapsibility and could be applied routinely with significant interrater reliability [21].
This entry is adapted from the peer-reviewed paper 10.3390/jcm13010165
References
- Chang, J.L.; Goldberg, A.N.; Alt, J.A.; Ashbrook, L.; Auckley, D.; Ayappa, I.; Bakhtiar, H.; Barrera, J.E.; Bartley, B.L.; Billings, M.E.; et al. International consensus statement on obstructive sleep apnea. Int. Forum. Allergy Rhinol. 2022, 13, 1061–1482.
- Bosi, M.; De Vito, A.; Gobbi, R.; Poletti, V.; Vicini, C. The importance of obstructive sleep apnoea and hypopnea pathophysiology for customized therapy. Eur. Arch. Otorhinolaryngol. 2017, 274, 1251–1261.
- Rotenberg, B.W.; Murariu, D.; Pang, K.P. Trends in CPAP adherence over twenty years of data collection: A flattened curve. J. Otolaryngol. Head Neck Surg. 2016, 45, 43.
- De Vito, A.; Woodson, B.T.; Koka, V.; Cammaroto, G.; Iannella, G.; Bosi, M.; Pelucchi, S.; Filograna-Pignatelli, G.R.; El Chater, P.; Vicini, C. OSA Upper Airways Surgery: A Targeted Approach. Medicina 2021, 57, 690.
- Pringle, M.B.; Croft, C.B. A comparison of sleep nasendoscopy and the Muller manoeuvre. Clin. Otolaryngol. Allied. Sci. 1991, 16, 559–562.
- Carrasco-Llatas, M.; Zerpa-Zerpa, V.; Dalmau-Galofre, J. Reliability of drug-induced sedation endoscopy: Interobserver agreement. Sleep Breath 2017, 21, 173–179.
- De Vito, A.; Carrasco Llatas, M.; Ravesloot, M.J.; Kotecha, B.; De Vries, N.; Hamans, E.; Maurer, J.; Bosi, M.; Blumen, M.; Heiser, C.; et al. European position paper on drug-induced sleep endoscopy: 2017 Update. Clin. Otolaryngol. 2018, 43, 1541–1552.
- Van de Perck, E.; Heiser, C.; Vanderveken, O.M. Concentric vs Anteroposterior-Laterolateral Collapse of the Soft Palate in Patients with Obstructive Sleep Apnea. Otolaryngol. Head Neck Surg. 2022, 166, 782–785.
- Olszewska, E.; Woodson, B.T. Palatal anatomy for sleep apnea surgery. Laryngoscope Investig. Otolaryngol. 2019, 4, 181–187.
- Migueis, D.P.; Thuler, L.C.; Lemes, L.N.; Moreira, C.S.; Joffily, L.; Araujo-Melo, M.H. Systematic review: The influence of nasal obstruction on sleep apnea. Braz. J. Otorhinolaryngol. 2016, 82, 223–231.
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004.
- Vanderveken, O.M.; Maurer, J.T.; Hohenhorst, W.; Hamans, E.; Lin, H.S.; Vroegop, A.V.; Anders, C.; de Vries, N.; Van de Heyning, P.H. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J. Clin. Sleep Med. 2013, 9, 433–438.
- Huyett, P.; Kent, D.T.; D’Agostino, M.A.; Green, K.K.; Soose, R.J.; Kaffenberger, T.M.; Woodson, B.T.; Huntley, C.; Boon, M.S.; Heiser, C.; et al. Drug-Induced Sleep Endoscopy and Hypoglossal Nerve Stimulation Outcomes: A Multicenter Cohort Study. Laryngoscope 2021, 131, 1676–1682.
- Woodson, B.T.; Robison, S.; Lim, H.J. Transpalatal advancement pharyngoplasty outcomes compared with uvulopalatopharyngoplasty. Otolaryngol. Head Neck Surg. 2005, 133, 211–217.
- Agrawal, V.K.; Kodur, S.; Jha, R.H. A Novel Grading System for Salpingopharyngeal Fold Hypertrophy in Obstructive Sleep Apnoea. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 60–65.
- Justin, G.A.; Chang, E.T.; Camacho, M.; Brietzke, S.E. Transoral Robotic Surgery for Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Otolaryngol. Head Neck Surg. 2016, 154, 835–846.
- Ilea, A.; Timuș, D.; Höpken, J.; Andrei, V.; Băbțan, A.M.; Petrescu, N.B.; Câmpian, R.S.; Boșca, A.B.; Șovrea, A.S.; Negucioiu, M.; et al. Oral appliance therapy in obstructive sleep apnea and snoring-systematic review and new directions of development. CRANIO 2021, 39, 472–483.
- Bae, M.R.; Chung, Y.S. Epiglottic collapse in obstructive sleep apnea. Sleep Med. Res. 2021, 12, 15–19.
- He, J.; Wang, C.; Li, W. Laryngopharyngeal Reflux in Obstructive Sleep Apnea-Hypopnea Syndrome: An Updated Meta-Analysis. Nat. Sci. Sleep. 2022, 14, 2189–2201.
- Yu, J.L.; Thuler, E.; Seay, E.G.; Schwartz, A.R.; Dedhia, R.C. The Accuracy and Reliability of Visually Assessed Pharyngeal Opening Pressures during Drug-Induced Sleep Endoscopy. Otolaryngol. Head Neck Surg. 2022, 168, 1945998221120793.
- Dedhia, R.C.; Seay, E.G.; Schwartz, A.R. Beyond VOTE: The New Frontier of Drug-Induced Sleep Endoscopy. ORL J. Otorhinolaryngol. Relat. Spec. 2022, 84, 296–301.
This entry is offline, you can click here to edit this entry!
