Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Berberine (BBR) is an isoquinoline alkaloid that can be extracted from herbs such as Coptis, Phellodendron, and Berberis. BBR has been widely used as a folk medicine to treat various disorders. It is a multi-target drug with multiple mechanisms. Studies have shown that it has antioxidant and anti-inflammatory properties and can also adjust intestinal microbial flora.
- berberine
- type 2 diabetes
- insulin
- inflammation
- oxidative stress
1. Introduction
Type 2 diabetes mellitus (T2DM), one of the most widespread persistent diseases, is characterized by hyperglycemia. Its incidence rate is constantly increasing. In 2021, 529 million people suffered from diabetes. It is predicted that this number will reach 1.3 billion by 2050 [1]. Prescription drugs and insulin supplementation represent the current primary treatment of T2DM; however, some adverse effects have been noted, including liver difficulties and lactic acidosis [2]. Consequently, an ongoing search for alternative medicines and herbal remedies for T2DM with high efficacy and low toxicity is essential.
Berberine (BBR) is an isoquinoline alkaloid compound that can be isolated from Coptis, Phellodendron, and Berberis. BBR has been used for many years as a folk medicine in China in treating diarrhea and diabetes [3]. It is also known for its anticancer, anti-inflammatory, and antibacterial effects [4]. BBR also has an antidiabetic impact similar to metformin [5]. In addition to T2D, BBR has a beneficial effect on the complications of diabetes, including renal damage [6], neuropathy [7], retinopathy [8], cognitive problems [9], cardiovascular complications [10], and endothelial dysfunction [11], through various mechanisms. Furthermore, many clinical studies have confirmed the beneficial effects of BBR in diabetic patients. Therefore, BBR can be used as a treatment in diabetic patients
2. Berberine in Diabetes and Related Complications Treatment
2.1. In Vitro Models of Diabetes Mellitus (DM)
One study investigated the effects of short-term treatment with BBR on 3T3-L1 adipocytes and L6 myoblasts. BBR inhibited triglyceride accumulation in fully differentiated and undifferentiated adipocytes. In addition, it reduced adipogenic gene expression and levels of most lipogenic transcripts (including the Fas receptor, also known as APO-1 or CD95), adipocyte determination and differentiation–dependent factor 1/sterol regulatory element–binding protein 1c (ADD1/SREBP1c), peroxisome proliferator-activated receptors (PPARs), CCAAT/enhancer binding proteins (C/EBPs), 11beta-hydroxysteroid dehydrogenase 1 (11β-HSD1), and adipocyte protein 2 (aP2). BBR increased the phosphorylation of AMP-activated protein kinase (AMPK) and ACC (a major substrate of AMPK) in both adipocytes and myoblasts.
Another laboratory investigation was conducted on neonatal rat cardiomyocytes exposed to hypoxia/reoxygenation with elevated glucose levels. BBR, at a concentration of 50 µM, decreased hypoxia/reoxygenation-promoted myocardial cell death, increased the Bcl-2/Bax ratio, decreased caspase-3 expression, activated phosphoinositide 3-kinase (PI3K)–Akt, and amplified AMP-activated protein kinase (AMPK) and endothelial nitric oxide synthase (eNOS) phosphorylation. The fact that prior treatment with either the PI3K/Akt inhibitor wortmannin or the AMPK inhibitor compound C decreased the antiapoptotic effect of BBR supported the mechanisms of BBR [12]. In the same in vitro study, BBR and metformin, either alone or in combination, were tested on a high-glucose-induced hepatocellular carcinoma (HepG2) cell line in order to evaluate the effects of both on lipid levels [13].
HepG2 cells were treated with glucose (33 mM) for 24 h after being pretreated with BBR and metformin. Concentrations of 20 and 40 μM of BBR could reduce the total lipid content and triglycerides in the treated HepG2 cells. Synergistic effects in reducing total lipid contents and triglyceride levels in HepG2 cells were obtained following the simultaneous administration of metformin and BBR at ratios of 2:10, 1:10, 0.5:10, and 0.25%. Furthermore, the combination of metformin and BBR at the lowest concentrations (0.25 and 5 μM) also showed a synergistic effect and reduced the expression of FAS and SREBP-1c [13].
2.2. Animal Models of Diabetes Mellitus (DM)
The promising antidiabetic effects of BBR have been reported in several animal models of DM, including streptozotocin (STZ) and alloxan-induced DM (Table 1).
Table 1. Protective effects of berberine in animal models of DM.
| Type of Extract or Constituent | Dose/Concentration | Study Model | Results | Ref. |
|---|---|---|---|---|
| Berberine chloride | 50 mg/kg/day; orally for 45 days | STZ-induced diabetic rats | ↓ Blood glucose and HbAlc ↑ Plasma insulin, hemoglobin, and body weight ↑ Pancreatic levels of SOD, CAT, GPx, GSH, vitamin E, and vitamin C ↓ LOOH and TBARS ↓ TNF-a, NF-kB, phospho-NF-kB-p65, COX-2, and iNOS ↓ Caspase-8, t-Bid, Bax, cytochrome-c, and cleaved caspase-3 ↑ Bcl-2 ↑ Anti-inflammatory mediator IL-10 and GLUT-2 |
[14] |
| 187.5 and 562.5 mg/kg; orally for 4 weeks | STZ-induced DM in rats | ↓ FBG, TGs, TC, FFAs, and apolipoprotein B ↑ HDL and apolipoprotein AI |
[15] | |
| 100 mg/Kg per day; intragastrically for 6 weeks 10 mg/Kg/d; intraperitoneally for 4 weeks |
STZ-induced DM in mice | ↓ FINS, HOMA-IR, and FPG, and expression of TLR4, TNF-α, IL-1β and IL-6 ↓ Pathological damage and macrophage (MΦ) infiltration in pancreatic islets of diabetic mice Regulated the probiotics in the intestinal tract Blocked the nuclear translocation of NF-κB in THP-1-derived MΦs |
[16] | |
| 156 mg/kg per day; intragastrically for 12 weeks | STZ-induced DM in rats | ↓ FINS, HOMA-IR, hyperlipidemia ↑ p-TORC2 levels Up-regulated expression of liver kinase B1, AMPK, and phosphorylated AMPK Down-regulated expression of the key gluconeogenic enzymes Inhibited TORC2 nuclear translocation in the liver tissues |
[17] | |
| Diabetic rats: 75 and 150 mg/kg/day; orally twice a day for 15 days Diabetic mice: 200 mg/kg/day; orally for 3 weeks |
STZ-induced DM in rats and KK-Ay diabetic mice | ↓ FBG and FINS ↑ Expression of insulin receptor mRNA and PKC |
[18] | |
| 150 mg/kg/d; orally for 9 weeks | STZ-induced T2D hamsters | ↑ Expression of LXRs and PPARs ↓ Expression of SREBPs in visceral white adipose tissue ↓ Body weight, total visceral white adipose tissue weight, blood glucose, FFAs, TC, LDL-c, and TGs ↑ Serum adiponectin ↓ Serum leptin, TNF-a, IL-6, and HOMA-IR ↓ Adipocyte size |
[19] | |
| 100 mg/kg/d; orally for 7 weeks | STZ-induced diabetic rats | ↓ FBG, plasma-free fatty acids, CRP, TGs, and TC Improved glucose tolerance Inhibited DPP-4 and PTP-1B activities Moderately improved glucose homeostasis |
[20] | |
| 5 mg/kg/day; intraperitoneally for 3 weeks | ob/ob and STZ-induced diabetic mice | Improved insulin, glucose tolerance, and glucose metabolism ↓ Blood glucose levels, cAMP, hepatic gluconeogenesis, and gluconeogenic gene expression Suppressed glucagon-induced CREB phosphorylation |
[21] | |
| 5 mg/kg/day; intraperitoneally for 3 weeks | db/db mice | ↓ Body weight ↓ Fat mass and the size of fat cells Food intake did not change Improved glucose tolerance |
[22] | |
| 100 mg/kg/d; orally for 2 weeks | db/db mice | Improved insulin resistance ↓ FBG Suppressed protein tyrosine phosphatase 1B ↑ Phosphorylation of insulin receptor, insulin receptor substrate1, and Akt |
[23] | |
| Berberine 100mg/kg/d and Berberine 100 mg/kg/d+ stachyose 200 mg/kg/d; orally for 55 days |
db/db mice | Improved glucose metabolism, the balance of α- and β-cells, and mucin-2 expression Increased abundance of Akkermansia muciniphila ↑ Fumaric acid level ↓ Metabolite all-transheptaprenyl diphosphate |
[24] | |
| 300 mg/kg/day; orally for 12 weeks | Alloxan-induced diabetic mice with renal injury | ↓ NF-κB, and the ↑ IκB-α ↓ Levels of fibronectin, transforming growth factor-beta 1, and intercellular adhesion molecule-1 |
[25] | |
| 380 mg/day; orally for 2 weeks | HFD-fed rats | ↓ Body weight, plasma triglycerides, and insulin resistance | [22] | |
| Dihydroberberine | 100 mg/kg/day; orally for 2 weeks | HFD-induced insulin resistance in mice and rat | Improved effectiveness of BBR Better oral bioavailability than BBR ↓ Augmented adiposity, TGs, and insulin resistance |
[26] |
| 5, 10 mg/kg/day; intraperitoneal injections for 4 weeks | HFD-fed mice | ↓ Insulin resistance, body weight, and HOMA-IR ↑ Synthesis of liver glycogen and SIRT1 expression Regulated SIRT1/FOXO1 pathway |
[27] | |
| 100 mg/kg/day; orally for 4 weeks | Mitochondria isolated from the liver of HFD-fed rats | ↑ Mitochondrial SirT3 activity Improved mitochondrial function Prevented a state of energetic deficit |
[28] | |
| Berberine | RB 0.7 (RB-L), 2.11 (RB-M), or 6.33 mg/kg/day (RB-H); orally for 8 weeks | High-sugar, high-fat diet (HSHFD)-induced diabetic KKAy mice | Improved glucolipid metabolism, insulin resistance, OGTT, insulin tolerance test (ITT), and pathological changes in the pancreases and livers of mice ↓ FBG, white fat index, TGs, LDL, GIP, and insulin level ↑ GLP-1, HDL, and glycogen content in the liver and muscle ↑ p-PI3K and p-AKT levels ↓ TXNIP expression |
[29] |
| 150 and 300 mg/kg/day; gavage for 12 weeks | HFD-fed rats | ↓ Body weight, urine volume, FBG, BUN, cholesterol, hepatic index levels, pathologic changes, and IR Improved albumin levels, glucose consumption, uptake, and inflammation ↑ Expression of PPM1B, PPARγ, LRP1, GLUT4, IRS-1, IRS-2, PI3K, AKT, and IKKβ Inhibited the phosphorylation of pIKKβ Ser181, total IKKβ, NF-κB p65, and JNK |
[30] | |
| Berberine 100 mg/kg/d for 30 days then 150 mg/kg/d; berberine combined with stachyose; BBR 100 mg/kg/d + stachyose 200 mg/kg/d for 30 days then BBR 150 mg/kg/d + 300 mg/kg/d; 69 days in total |
Zucker diabetic fatty rats | ↓ Blood glucose Improved impaired glucose tolerance ↑ Abundance of beneficial Akkermansiaceae, ↓ Abundance of pathogenic Enterobacteriaceae, Desulfovibrionaceae, and Proteobacteria ↓ Expression of intestinal Egr1 and Hbegf ↑ Expression of miR-10a-5p (just combination therapy) |
[31] |
2.2.1. Protective Effects of Berberine against db/db and STZ-Induced DM
In STZ-induced diabetic rats, BBR exhibited antihyperglycemic, antioxidant, anti-inflammatory, and antiapoptotic activities. For example, in one study, a BBR chloride treatment (50 mg/kg/day) was administered orally to diabetic rats for forty-five days. When administered orally, BBR chloride significantly reduced blood glucose levels and HbAlc, while it increased plasma insulin, hemoglobin, and body weight. In addition, the pancreatic levels of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), reduced glutathione (GSH), vitamin E, and vitamin C increased, while those of lipid peroxidation markers, i.e., lipid hydroperoxides (LOOHs) and 2-thiobarbituric acid reactive substances (TBARSs), decreased.
It was shown that BBR reduced fasting plasma glucose (FPG); fasting insulin (FINS); the expression of TLR4, TNF-α, IL-1β and IL-6; pathological damage; and macrophage (MΦ) infiltration in pancreatic islets of diabetic mice. It also regulated probiotics in the intestinal tracts of diabetic mice. Furthermore, BBR blocked the nuclear translocation of NF-κB in THP-1-derived MΦs. Therefore, BBR plays a crucial role in regulating the gut microbiota and inhibiting the TLR4-NF-κB signaling pathway, and, through these mechanisms, it can suppress inflammation and alleviate symptoms related to diabetes [16]. It should be mentioned that the anti-inflammatory mechanism of BBR is to inhibit the binding between Toll-like receptor 4 (TLR4) and MyD88 and disturb the TLR4/MyD88/NF-κB signaling pathway [32].
Another study demonstrated that BBR (5 mg/kg/day) reduced blood glucose levels; improved insulin activity, glucose tolerance, and glucose metabolism; and decreased hepatic gluconeogenesis in the livers of ob/ob and STZ-induced diabetic mice. In addition, it decreased glucagon-induced glucose production and gluconeogenic gene expression in hepatocytes, apparently through cAMP reduction, and also suppressed glucagon-induced CREB phosphorylation [21].
2.2.2. Protective Effects of Berberine against Alloxan-Induced DM
In alloxan-induced diabetic mice with renal impairment, nuclear factor-kappa B (NF-κB) was reduced after treatment with BBR at 300 mg/kg/day. The reduced IB-level worsening was partially recovered. In contrast to the diabetic model group, BBR reduced fibronectin, transforming growth factor (TGF)-β1, and intercellular adhesion molecule-1 (ICAM-1). The inhibitory effects of berberine on the NF-κB signaling pathway may explain why it has an ameliorative impact on extracellular matrix formation [25].
2.2.3. Protective Effects of Berberine against HFD-Induced DM
A study demonstrated that oral administration of 380 mg/day of BBR to rats fed with high-fat food for two weeks reduced body weight. Furthermore, BBR reduced plasma triglycerides and insulin resistance in animals with a high fat content [22]. In another study, 100 mg/kg/day dihydro berberine (a BBR derivative) enhanced the in vivo efficacy of BBR in terms of suppression of increased adiposity, triglyceride accumulation in tissues, and insulin resistance. This finding is likely due to the optimized oral bioavailability of BBR [26].
Another study showed that 5 and 10 mg/kg/day of BBR administration for four weeks significantly reduced body weight, insulin, and the HOMA-IR index without altering food intake in high-fat diet-fed mice [27]. Another study demonstrated that oral administration of 100 mg/kg/day of BBR for four weeks reverted FBGs to normal levels and also decreased the levels of HbA1c, triglycerides and phospholipids, leptin, and insulin in high-fat diet-fed rodents [28].
2.3. Effects of Berberine on Insulin Resistance and Secretion
Interestingly, BBR acted like insulin and improved insulin resistance in db/db mice. It stimulated glucose uptake by 3T3-L1 adipocytes at concentrations of 1.25 and 2.5 µM and glucose uptake by L6 myocytes at 2.5–5 µM. BBR also suppressed the phosphatase activity of protein tyrosine phosphatase 1B. In addition, phosphorylation of insulin receptors, insulin receptor substrate1, and Akt were increased by BBR in 3T3-L1 adipocytes [23].
BBR increased the expression of insulin receptor proteins in cultured human liver cells (HepG2) and L6 myocytes through the activation of the insulin receptor gene promotor protein kinase C (PKC)-dependent promoter [18].
In HepG2 cells, BBR up-regulated the expression of alpha7 nicotinic acetylcholine receptor (α7nAChR) protein and suppressed the AChE enzyme. It also showed an anti-inflammatory effect by reducing the pIKKβ Ser181/IKKβ ratio, NF-κB p65 expression, and IL-6 levels. Through this mechanism, BBR could improve insulin resistance [33].
Using a dose-dependent approach, BBR inhibited respiration in L6 myotubes and muscle mitochondria, mirroring the effects of metformin and rosiglitazone on respiratory complex I. Through increased AMPK activity, Respiratory Complex I is a primary target for compounds that improve overall insulin sensitivity. AMPK activation by BBR was not contingent on LKB1 or CAMKKβ activity, indicating a primary regulation at the level of the AMPK phosphatase [26].
BBR improved the insulin resistance of visceral white adipose tissue in T2D hamsters by increasing liver X receptors (LXRs) and PPARs and decreasing sterol regulatory element-binding proteins (SREBPs) [19].
Administration of 100 mg/kg/day of BBR improved many factors associated with insulin resistance in STZ-induced diabetic rats. It was shown that BBR inhibited tyrosine phosphatase-1B (PTP-1B) and dipeptidyl peptidase-4 (DPP-4) dose-dependently, both of which have key roles in glucose metabolism. The IC50 values for DPP-4 and PTP-1B were 67 μM and 205 μM, respectively. Inhibition of DPP-4 and PTP-1B is thought to be involved in the insulin-sensitizing effect of BBR [20].
Using in vitro studies, BBR was shown to reduce leukotriene B4 (LTB4)-induced intracellular insulin resistance and inflammation in liver cells. It should also be noted that BBR reduced chemotaxis and inflammatory responses of LTB4-activated macrophages. There was a significant decrease in M1 macrophage gene expression by BBR, which was significantly increased by LTB4 [34]. The possible mechanism of BBR could be related to its effect on the LTB4–BLT1 axis, known as a target for treating metabolic diseases [35]. As a consequence of this interaction, BBR mediated the down-regulation of p-NF-κB expression in macrophages caused by LTB4 [34]. One of the mechanisms of the anti-inflammatory effect of BBR was the MyD88/NF-κB pathway [32]. The MyD88/NF-κB pathway has also been seen as the downstream pathway of the LTB4 pathway [36]. On the other hand, interestingly, LTB4 can influence insulin signaling and inflammation through leukotriene B4 receptor 1 (BLT1), which is often expressed on the surface of liver cells and macrophages. Indeed, the inhibition of BLT1 could suppress the chemotaxis and tracking of macrophages and other immune cells in metabolic tissue, as well as the development of inflammation–insulin resistance syndrome [34].
2.4. Protective Effects of Berberine against Diabetes Complications
DM is an endocrine disorder that can lead to many chronic complications, including osteoporosis, retinopathy, nephropathy, neuropathy, cardiovascular diseases, and hepatic disorders. Interestingly, protective properties of BBR against DM complications have been reported.
2.4.1. Diabetes-Induced Osteoporosis
One of the complications of diabetes mellitus is osteoporosis. Diabetes suppresses osteogenesis and compromises the osseointegration process, causing dental-implant failure. Dental implants are used worldwide to treat dentition defects, but there is a major problem for diabetic patients [37].
To study the effect of BBR on implant recovery, 120 mg/kg/day of BBR was administrated (gated for four weeks) to STZ-induced diabetic rats and was also added to high-density bone mesenchymal stem cells (BMSCs) with medium glucose contents. The results showed that BBR improved glucose and bone metabolism in diabetic rats through the ROS-mediated IRS-1 signaling pathway.
2.4.2. Diabetes-Induced Gut Microbiota Alteration
The microbiota is considered a functional organ, so its composition affects the host’s glycemic control system [38]. For example, one of the microbiota components related to diabetic chronic inflammation is lipopolysaccharide (LPS), a component of Gram-negative bacteria cell walls [3].
Treatment with 100 mg/kg/day of BBR for three weeks inhibited the progression from prediabetes to diabetes in 70% of diabetic fatty rats by restoring an average diversity of gut microbiota and increasing fasting plasma GLP-2 and glutamine-induced intestinal GLP-2 secretion. In this experiment, BBR reduced food intake, FBG, insulin resistance, and LPS levels but increased the number of goblet cells and villi length. Furthermore, it increased the expression of mucins and major tight junction proteins, namely, occludin and zona occludens-1 (ZO-1), and down-regulated the expressions of TLR4, NF-κB, and TNF-α [39].
2.4.3. Diabetic-Induced Hepatic Damage
An experiment was conducted to determine whether BBR could ameliorate T2D-associated hepatic gluconeogenic and lipid metabolism disorders in STZ-induced diabetic mice and palmitic acid-treated HepG2 cells. BBR treatment reduced levels of HNF-4α and expression of miR122; the key gluconeogenesis enzymes, namely, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase); and key enzymes and proteins in lipid metabolism, such as SREBP-1, fatty acid synthase-1 (FAS-1), and acetyl-coenzyme A carboxylase (ACCα), but increased carnitine palmitoyltransferase-1 (CPT1) in both diabetic mice and palmitic acid-treated HepG2 cells. MicroRNA 122 is an essential hepatocyte nuclear factor 4α (HNF4α) regulator in the regulation of hepatic gluconeogenesis and lipid metabolism in HepG2 cells. So, the protective effect of BBR on hepatic gluconeogenesis and lipid metabolism disorders was mediated by HNF-4α and maintained downstream of miR122 [40].
2.4.4. Diabetic Retinopathy
BBR showed a protective effect against diabetic retinopathy by activating the AMPK/mTOR signaling pathway [41][42] and inhibiting Akt/mTOR-mediated hypoxia-inducible factor (HIF)-1α/vascular endothelial growth factor (VEGF) activation [8]. In diabetic patients, normal LDL changes to highly oxidized and glycated (HOG)-LDL. This type of LDL damages retinal cells, leading to diabetic retinopathy [43]. In an in vitro study, BBR was added to HOG-LDL-induced human retinal Muller cells. The results showed that BBR activates AMPK and could increase cell viability (reducing HOG-LDL-induced cytotoxicity, autophagy, and apoptosis). BBR decreases oxidative stress, the expression of angiogenic factors, inflammation, and glial fibrillary acidic (GFA) protein expression. In conditions of retinal damage, GFA is over-expressed; therefore, it is considered a significant factor in the development of retinopathy [42].
2.4.5. Diabetic Vascular Complications
Endothelial dysfunction plays a major role in the onset of vascular problems due to diabetes. Advanced glycation end products (AGEs) have been linked to endothelial dysfunction through various mechanisms and excessive glucose [44][45].
To simulate clinical circumstances, researchers created an in vitro model of diabetic micro-endothelial (microEC) damage caused by the combination of high glucose and AGEs. The results showed that BBR treatments significantly increased the synthesis of thrombomodulin, NOS, and NO. Additionally, BBR was found to have potent inhibitory effects on AGE production [46].
In cultured endothelial cells and blood vessels isolated from rat aorta, BBR ameliorated high-glucose-induced endothelial dysfunction by increasing eNOS and NO and decreasing glucose-induced ROS, cell apoptosis, NF-kB activation, and the expression of adhesion molecules, which led to inhibition of the attachment of monocytes to endothelial cells. Furthermore, BBR increased endothelium-dependent vasodilatation through activation of AMPK [47].
2.4.6. Diabetic-Induced Neuropathy
BBR improved cold and mechanical allodynia at doses of 10 and 20 mg/kg (single and repeated intraperitoneal injection, twice daily for 14 days) in a rat diabetic neuropathy model. A dose of 5 mg/kg of BBR was insufficient to significantly reduce allodynia. Diabetes increased hepatic MDA, SOD, catalase, and GPx activities, while BBR administration reduced all of these factors in a dose-dependent manner. The antioxidative effects of 10 mg/kg BBR were quite similar to those of 10 mg/kg amitriptyline. A dosage of BBR 20 mg/kg showed antiallodynic results identical to those obtained with a dosage of 10 mg/kg amitriptyline. Therefore, the antiallodynic effect of BBR is assumed to be related to its antioxidative effects [13]. In another study, BBR improved mechanical allodynia and thermal hyperalgesia by developing a mechanical threshold and thermal latency in STZ-induced diabetic mice. It inhibited the activations of microglia and astrocytes in the spinal cord and also inhibited the expression of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) and inflammatory proteins (iNOS and COX-2). Therefore, the mechanism of the antinociceptive effect of BBR on diabetic neuropathic pain is related to its ability to suppress neuroglia activation and inflammation [48].
2.4.7. Diabetic-Induced Nephropathy
Kumaş et al. [49] investigated the effect of 50, 100, and 150 mg/kg/day of BBR on STZ-induced diabetic rats with renal ischemia/reperfusion injury. The results showed that the dosage of 50 mg/kg/day was insufficient to produce sufficient protective effects. Dosages of 100 and 150 mg/kg/day significantly improved renal function and reduced the elevation of BUN and creatinine levels. BBR rearranged the intercellular ion concentration by increasing the decreased activity of Ca2+-ATPase and Na+/K+-ATPase enzymes in diabetic rats. All doses of BBR (50, 100, and 150 mg/kg/day) reduced enzyme lactate dehydrogenase (LDH) levels, a marker of tubular necrosis. In general, BBR improves diabetic-induced nephropathy through its antioxidant, anti-inflammatory, and antiapoptotic properties [49].
BBR doses of 100 mg/kg and 200 mg/kg were administered to STZ-induced diabetic nephropathic golden hamsters for eight weeks. Consequently, blood glucose, blood lipids, and renal function were improved and the expression of inflammatory factors (IL-1β and IL-6), NOD-like receptor pyrin domain-containing protein 3 (NLRP3), caspase-1, and Gasdermin D (GSDMD); the number of TUNEL-positive cells; and MDA levels were decreased; however, Nrf2 expression was increased [50]. Briefly, BBR inhibited pyroptosis and diabetic nephropathic damage by regulating Nrf2 and NLRP3-Caspase-1-GSDMD signaling [50].
2.4.8. Diabetic-Induced Cardiovascular Disease
To investigate the cardioprotective effect of BBR against ischemia/reperfusion (I/R) in diabetic rats, diabetes was induced in rats for 12 weeks. Then, saline or BBR (100, 200, and 400 mg/kg/d) was administered intragastrically to diabetic rats, starting from weeks 9 to 12. At the end of the period of 12 weeks, myocardial ischemia and reperfusion were induced in all rats. The results showed that BBR significantly helped the recovery of systolic/diastolic cardiac function and reduced myocardial apoptosis through the activation of AMPK and PI3KAkt–eNOS signaling in the mentioned animals [12]. As in the above study, 100 mg/kg BBR was administered to STZ-induced diabetic rats for seven days before the I/R induction. This BBR pretreatment reduced I/R injury and decreased arrhythmia in diabetic rats. BBR reduced the serum levels of TGs, TC, and MDA, while it did not change the serum levels of FBG and SOD. This study demonstrated that the cardioprotective mechanism of BBR leads to the inhibition of glycogen synthase kinase 3β (GSK3β) and increases AKT phosphorylation and AMP/ATP and ADP/ATP ratios, which in turn lead to an increase in AMPK in non-ischemic areas [51].
In another study, diabetes mellitus was induced in pregnant mice and then the effect of BBR on the function of the cardiac mitochondria and mitochondrial phospholipid cardiolipin of the newborn mice was investigated. To induce gestational diabetes, female mice were fed a high-fat diet for six weeks before reproduction. Another group of pregnant mice was fed a low-fat diet. Lean male offspring and male offspring exposed to gestational diabetes were randomly assigned to a low-fat diet, a high-fat diet, or a high-fat diet containing BBR (160 mg/kg/day) at weaning for 12 weeks. The results showed that the expression of the cardiolipin remodeling enzyme (tafazzin) increased in male offspring exposed to gestational diabetes and fed a diet containing BBR, followed by an increase in the amount of tetra-linoleic-cardiolipin and total cardiolipin in the heart. These descendants also showed increased expression of cardiac enzymes involved in fatty acid uptake, oxidation, and electron transport chain subunits.
2.4.9. Diabetes-Induced Central Nervous System (CNS) Disorders
Chronic inflammation and mediators of insulin resistance cause the production of β-amyloid (Aβ)42, a marker of Alzheimer’s disease, in the diabetic brain [9]. Furthermore, memory impairment in diabetes mellitus was mainly associated with glucose uptake/metabolism in the medial prefrontal cortex (mPFC). Intragastric administration of BBR (187.5 mg/Kg/d) ameliorated diabetes-associated memory impairment by modulating the abnormal inflammatory response and improving insulin resistance in the mPFCs of STZ-induced diabetic rats. BBR also reduced the activation of the PI3K/Akt/mTOR and MAPK signaling pathways, as well as two isoforms, PKCη and PKCε, and the translocation of NF-κB in neurons. In addition, GLUT3 was significantly increased in the BBR-treated animals. Furthermore, BBR increased glucose uptake, while decreasing the expressions of amyloid precursor protein and β-site amyloid-precursor-protein-cleaving enzyme 1 (BACE-1 and β-secretase 1) and the production of oligomeric Aβ42. Therefore, BBR has the potency to accelerate information consolidation and improve cognitive impairment in diabetes [9].
In STZ-induced Alzheimer’s diabetic rats, BBR alleviated memory impairment by suppressing the ER stress pathway. It decreased major ER stress-related proteins in the hippocampus, eliminated Aβ deposition, restored the disorganized arrangement and damage of nerve cells, and reduced the apoptosis rate of nerve cells, leading to improvement in diabetic Alzheimer’s disease [52]. A dosage of 187.75 mg/kg/day of BBR improved spatial learning memory by inhibiting Aβ formation and reducing CSF/glycemia, inflammatory response, and AChE activity. BBR has been found to increase the expression of α7-nAChRs, inhibiting CNS or peripheral inflammation [53]. Doses of 20 and 40 mg/kg of BBR showed neuroprotective effects against neonatal-STZ-induced diabetic peripheral neuropathy through a reduction in pro-inflammatory cytokines and oxide-nitrosative stress and an increase in the expression levels of BDNF, IGF-1, PPAR-©, and AMPK. Through these mechanisms, BBR ameliorated impaired allodynia, hyperalgesia, and impaired nerve conduction velocity in neonatal diabetic rats with neuropathy [54][55].
2.5. Clinical Investigations
A clinical trial was conducted with 97 T2DM patients. Fifty patients received BBR (1 g/d orally), twenty-six patients received metformin (1.5 g/d orally), and the others (21 patients) received rosiglitazone (4 mg/d orally) for two months. BBR reduced FBG and HbA1c, as did metformin and rosiglitazone. It reduced TGs more than metformin and rosiglitazone. BBR also decreased the FINS. No adverse effects were observed in the BBR group. Furthermore, blood samples from the BBR group showed a high number of lymphocytes expressing insulin receptors [5].
In another series of experiments, 35 patients with chronic hepatitis and T2DM or impaired fasting glycemia were enrolled. Eighteen patients were infected with HCV, and seventeen had HBV disease. Again, all 35 patients were administered 1g/d of BBR for two months. The results showed that BBR reduced all patients’ FBG, TG, ALT, and AST levels. Furthermore, no adverse effects were observed in the BBR group [5].
In a randomized, double-blinded, placebo-controlled study, 365 participants with T2D were enrolled and randomly divided into four groups: the first group received a BBR dose of 0.6 g (six pills twice a day) plus 4 g of probiotics (two strips of powder once a day) (Prob + BBR), the second group received probiotics plus placebo (Prob), the third group received BBR plus placebo (BBR), and the fourth received placebo plus placebo (Plac) for 12 weeks. The results showed that postprandial TC and LDL levels were reduced more significantly in the Prob+BBR group than in BBR or Prob alone. Furthermore, several types of postprandial lipidomic metabolites were reduced: medium-chain fatty acids (FFAs), acyl-carnitines, and multiple glycerophospholipids: lysoglycerophosphatidylcholine (LPC), lysoglycerophatidylethanolamine (LPE), glycerophosphatidylcholine (PC), and glycerophatidylethanolamine (PE) with alkyl and alkenyl substituents. Fecal samples from the participants showed changes in fecal Bifidobacterium breve abundance that could be related to the therapeutic effects of BBR and Prob+BBR. In vitro analysis showed that BBR activated four fadD genes encoding long-chain Acyl-CoA synthetase in the B. breve strain. Consistent with this, BBR reduced the concentration of FFAs in the medium of B. breve, which may be related to its effect on fadD genes. Therefore, BBR reduced intraluminal lipids for absorption and synergized with Prob [56].
2.6. Toxicity of and Cautionary Notes on Berberine
BBR toxicity varies depending on the amount of BBR contained in a compound, the route of administration, and the type of organism. When orally administered to mice, Berberis vulgaris root powder had an LD50 value of 2600 mg/kg, B. vulgaris root extract had an LD50 value of 520 mg/kg, and pure BBR had an LD50 value of 329 mg/kg. When administered intraperitoneally to mice, pure BBR had an LD50 value of 23 mg/kg [57].
In rats, the LD50 value of B. vulgaris after oral treatment of the root extract fraction was 1,280 mg/kg, and the LD50 value of BBR sulfate after IP treatment of the BBR sulfate extracted from Berberis aristate was 205 mg/kg. Moreover, 40% of rats experienced diarrhea after receiving 50 mg/kg of BBR sulfate, resulting in an immediate negative impact on the digestive system [57].
Oral administration of 100 mg/kg BBR to cats caused vomiting for 6–8 h, while 100 mg/kg BBR administration for 8–10 days caused the death of all animals. Oral administration of berberine sulfate in cats at doses of 50 or 100 mg/kg for 10 days caused inflammatory bleeding problems in both the small and large intestines [58]. Dogs showed some moderate signs of toxicity at low doses of BBR and related compounds. These signs included salivation, nausea, diarrhea, emesis, muscle tremor, and occasional paralysis [58].
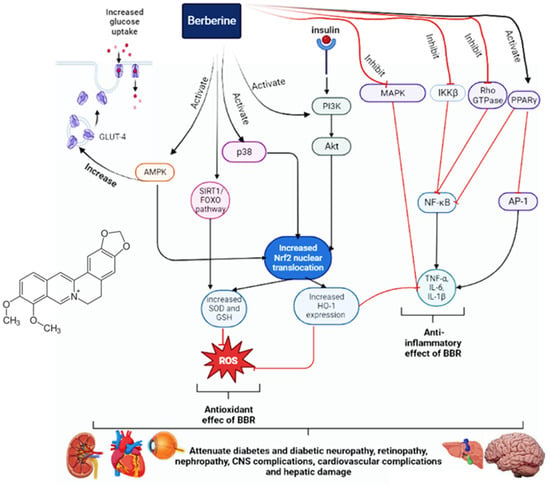
3. Conclusions
BBR is an isoquinoline alkaloid with anticancer, anti-inflammatory, antioxidant, and antimicrobial properties. It has proven beneficial in the treatment of diabetes and its complications, such as neuropathy, nephropathy, retinopathy, cardiomyopathy, osteoporosis, hepatic damage, endothelial dysfunction, and vascular problems. Most of the research concerns the effect of BBR on insulin resistance and secretion. BBR can increase insulin resistance and reduce its secretion by enhancing the expression of insulin receptors, Akt, and AMPK and reducing NF-κB. Since inflammation is a cause of type 2 diabetes (T2D), BBR can stop the development of T2D due to its anti-inflammatory properties. The use of BBR can positively influence diabetes-induced arrhythmia by shortening the prolonged QTc interval and restoring the diminished K+ current and L-type Ca2+ current to their normal states.
Furthermore, it can also reduce diabetes-induced fibrosis and cardiac dysfunction. BBR has beneficial effects for diabetic patients with hypertension due to its influence on vascular relaxation. It can also decrease the activities of AChE, BChE, and MAO, thereby improving cognitive performance, learning, and memory in diabetic patients. Furthermore, it can inhibit neuronal apoptosis and improve diabetic retinopathy by reducing VEGF levels. It can reduce hepatic gluconeogenesis and disorders of lipid metabolism, as well as provide a protective effect on the liver. The main antidiabetic mechanisms and effects of BBR are illustrated in Figure 1.

Figure 1. The antidiabetic mechanisms of berberine.
This entry is adapted from the peer-reviewed paper 10.3390/ph17010007
References
- Friedman, J.M. Modern science versus the stigma of obesity. Nat. Med. 2004, 10, 563–569.
- Huang, M.; Wang, F.; Zhou, X.; Yang, H.; Wang, Y. Hypoglycemic and hypolipidemic properties of polysaccharides from Enterobacter cloacae Z0206 in KKAy mice. Carbohydr. Polym. 2015, 117, 91–98.
- Pang, B.; Zhao, L.-H.; Zhou, Q.; Zhao, T.-Y.; Wang, H.; Gu, C.-J.; Tong, X.-L. Application of berberine on treating type 2 diabetes mellitus. Int. J. Endocrinol. 2015, 2015, 905749.
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157.
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.-D.; Zhao, W.; Wang, Z.-Z.; Wang, S.-K.; Zhou, Z.-X.; Song, D.-Q.; Wang, Y.-M.; et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism 2010, 59, 285–292.
- Wu, D.; Wen, W.; Qi, C.-L.; Zhao, R.-X.; Lü, J.-H.; Zhong, C.-Y.; Chen, Y.-Y. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine 2012, 19, 712–718.
- Zhou, J.; Du, X.; Long, M.; Zhang, Z.; Zhou, S.; Zhou, J.; Qian, G. Neuroprotective effect of berberine is mediated by MAPK signaling pathway in experimental diabetic neuropathy in rats. Eur. J. Pharmacol. 2016, 774, 87–94.
- Wang, N.; Zhang, C.; Xu, Y.; Tan, H.-Y.; Chen, H.; Feng, Y. Berberine improves insulin-induced diabetic retinopathy through exclusively suppressing Akt/mTOR-mediated HIF-1α/VEGF activation in retina endothelial cells. Int. J. Biol. Sci. 2021, 17, 4316–4326.
- Chen, Q.; Mo, R.; Wu, N.; Zou, X.; Shi, C.; Gong, J.; Li, J.; Fang, K.; Wang, D.; Yang, D.; et al. Berberine ameliorates diabetes-associated cognitive decline through modulation of aberrant inflammation response and insulin signaling pathway in DM rats. Front. Pharmacol. 2017, 8, 334.
- Wang, L.; Yu, C.; Fu, Y.; Li, Q.; Sun, Y. Berberine elicits anti-arrhythmic effects via IK1/Kir2. 1 in the rat type 2 diabetic myocardial infarction model. Phytother. Res. 2011, 25, 33–37.
- Wang, C.; Li, J.; Lv, X.; Zhang, M.; Song, Y.; Chen, L.; Liu, Y. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur. J. Pharmacol. 2009, 620, 131–137.
- Chen, K.; Li, G.; Geng, F.; Zhang, Z.; Li, J.; Yang, M.; Dong, L.; Gao, F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K–Akt signaling in diabetic rats. Apoptosis 2014, 19, 946–957.
- Babaei Khorzoughi, R.; Namvarjah, F.; Teimouri, M.; Hosseini, H.; Meshkani, R. In-vitro synergistic effect of metformin and berberine on high glucose-induced lipogenesis. Iran. J. Pharm. Res. 2019, 18, 1921–1930.
- Chandirasegaran, G.; Elanchezhiyan, C.; Ghosh, K.; Sethupathy, S. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of Streptozotocin induced diabetic rats. Biomed. Pharmacother. 2017, 95, 175–185.
- Leng, S.-H.; Lu, F.-E.; Xu, L.-J. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol. Sin. 2004, 25, 496–502.
- Cao, W.; Hu, L.; Chen, H.; Gao, Y.; Liang, Y.; Wu, Y.; Yang, Q.; Tang, N.; Cao, J.; Xiao, J. Berberine alleviates chronic inflammation of mouse model of type 2 diabetes by adjusting intestinal microbes and inhibiting TLR4 signaling pathway. Int. J. Clin. Exp. Med. 2017, 10, 10267–10276.
- Jiang, S.-J.; Dong, H.; Li, J.-B.; Xu, L.-J.; Zou, X.; Wang, K.-F.; Lu, F.-E.; Yi, P. Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J. Gastroenterol. 2015, 21, 7777.
- Kong, W.-J.; Zhang, H.; Song, D.-Q.; Xue, R.; Zhao, W.; Wei, J.; Wang, Y.-M.; Shan, N.; Zhou, Z.-X.; Yang, P.; et al. Berberine reduces insulin resistance through protein kinase C–dependent up-regulation of insulin receptor expression. Metabolism 2009, 58, 109–119.
- Li, G.-S.; Liu, X.-H.; Zhu, H.; Huang, L.; Liu, Y.-L.; Ma, C.-M.; Qin, C. Berberine-improved visceral white adipose tissue insulin resistance associated with altered sterol regulatory element-binding proteins, liver X receptors, and peroxisome proliferator-activated receptors transcriptional programs in diabetic hamsters. Biol. Pharm. Bull. 2011, 34, 644–654.
- Chen, Y.; Wang, Y.; Zhang, J.; Sun, C.; Lopez, A. Berberine improves glucose homeostasis in streptozotocin-induced diabetic rats in association with multiple factors of insulin resistance. ISRN Endocrinol. 2011, 2011, 519371.
- Zhong, Y.; Jin, J.; Liu, P.; Song, Y.; Zhang, H.; Sheng, L.; Zhou, H.; Jiang, B. Berberine attenuates hyperglycemia by inhibiting the hepatic glucagon pathway in diabetic mice. Oxidative Med. Cell. Longev. 2020, 2020, 6210526.
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.-M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264.
- Chen, C.; Zhang, Y.; Huang, C. Berberine inhibits PTP1B activity and mimics insulin action. Biochem. Biophys. Res. Commun. 2010, 397, 543–547.
- Li, C.-N.; Wang, X.; Lei, L.; Liu, M.-Z.; Li, R.-C.; Sun, S.-J.; Liu, S.-N.; Huan, Y.; Zhou, T.; Liu, Q.; et al. Berberine combined with stachyose induces better glycometabolism than berberine alone through modulating gut microbiota and fecal metabolomics in diabetic mice. Phytother. Res. 2020, 34, 1166–1174.
- Liu, W.; Zhang, X.; Liu, P.; Shen, X.; Lan, T.; Li, W.; Jiang, Q.; Xie, X.; Huang, H. Effects of berberine on matrix accumulation and NF-kappa B signal pathway in alloxan-induced diabetic mice with renal injury. Eur. J. Pharmacol. 2010, 638, 150–155.
- Turner, N.; Li, J.-Y.; Gosby, A.; To, S.W.C.; Cheng, Z.; Miyoshi, H.; Taketo, M.M.; Cooney, G.J.; Kraegen, E.W.; James, D.E.; et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: A mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 2008, 57, 1414–1418.
- Sui, M.; Jiang, X.; Sun, H.; Liu, C.; Fan, Y. Berberine ameliorates hepatic insulin resistance by regulating microRNA-146b/SIRT1 pathway. Diabetes Metab. Syndr. Obes. 2021, 14, 2525–2537.
- Teodoro, J.S.; Duarte, F.V.; Gomes, A.P.; Varela, A.T.; Peixoto, F.M.; Rolo, A.P.; Palmeira, C.M. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: A possible role for SirT3 activation. Mitochondrion 2013, 13, 637–646.
- He, Q.; Chen, B.; Wang, G.; Zhou, D.; Zeng, H.; Li, X.; Song, Y.; Yu, X.; Liang, W.; Chen, H.; et al. Co-Crystal of Rosiglitazone With Berberine Ameliorates Hyperglycemia and Insulin Resistance Through the PI3K/AKT/TXNIP Pathway In Vivo and In Vitro. Front. Pharmacol. 2022, 13, 842879.
- Wu, Y.S.; Li, Z.M.; Chen, Y.T.; Dai, S.J.; Zhou, X.J.; Yang, Y.X.; Lou, J.S.; Ji, L.T.; Bao, Y.T.; Xuan, L. Berberine improves inflammatory responses of diabetes mellitus in zucker diabetic fatty rats and insulin-resistant HepG2 cells through the PPM1B pathway. J. Immunol. Res. 2020, 2020, 2141508.
- Li, C.; Cao, H.; Huan, Y.; Ji, W.; Liu, S.; Sun, S.; Liu, Q.; Lei, L.; Liu, M.; Gao, X.; et al. Berberine combined with stachyose improves glycometabolism and gut microbiota through regulating colonic microRNA and gene expression in diabetic rats. Life Sci. 2021, 284, 119928.
- Gong, J.; Li, J.; Dong, H.; Chen, G.; Qin, X.; Hu, M.; Yuan, F.; Fang, K.; Wang, D.; Jiang, S.; et al. Inhibitory effects of berberine on proinflammatory M1 macrophage polarization through interfering with the interaction between TLR4 and MyD88. BMC Complement. Altern. Med. 2019, 19, 314.
- Li, F.; Zhao, Y.-B.; Wang, D.-K.; Zou, X.; Fang, K.; Wang, K.-F. Berberine relieves insulin resistance via the cholinergic anti-inflammatory pathway in HepG2 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 64–69.
- Gong, M.; Duan, H.; Wu, F.; Ren, Y.; Gong, J.; Xu, L.; Lu, F.; Wang, D. Berberine Alleviates Insulin Resistance and Inflammation via Inhibiting the LTB4–BLT1 Axis. Front. Pharmacol. 2021, 12, 722360.
- Johnson, A.M.F.; Hou, S.; Li, P. Inflammation and insulin resistance: New targets encourage new thinking. BioEssays 2017, 39, 1700036.
- Sánchez-Galán, E.; Gómez-Hernández, A.; Vidal, C.; Martín-Ventura, J.L.; Blanco-Colio, L.M.; Muñoz-García, B.; Ortega, L.; Egido, J.; Tuñón, J. Leukotriene B4 enhances the activity of nuclear factor-κB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc. Res. 2009, 81, 216–225.
- Costantini, S.; Conte, C. Bone health in diabetes and prediabetes. World J. Diabetes 2019, 10, 421–445.
- Shi, Y.; Hu, J.; Geng, J.; Hu, T.; Wang, B.; Yan, W.; Jiang, Y.; Li, J.; Liu, S. Berberine treatment reduces atherosclerosis by mediating gut microbiota in apoE−/− mice. Biomed. Pharmacother. 2018, 107, 1556–1563.
- Wang, Y.; Liu, H.; Zheng, M.; Yang, Y.; Ren, H.; Kong, Y.; Wang, S.; Wang, J.; Jiang, Y.; Yang, J. Berberine slows the progression of prediabetes to diabetes in Zucker diabetic fatty rats by enhancing intestinal secretion of glucagon-like peptide-2 and improving the gut microbiota. Front. Endocrinol. 2021, 12, 609134.
- Wei, S.; Zhang, M.; Yu, Y.; Lan, X.; Yao, F.; Yan, X.; Chen, L.; Hatch, G.M. Berberine Attenuates Development of the Hepatic Gluconeogenesis and Lipid Metabolism Disorder in Type 2 Diabetic Mice and in Palmitate-Incubated HepG2 Cells through Suppression of the HNF-4α miR122 Pathway. PLoS ONE 2016, 11, e0152097.
- Chen, H.; Ji, Y.; Yan, X.; Su, G.; Chen, L.; Xiao, J. Berberine attenuates apoptosis in rat retinal Müller cells stimulated with high glucose via enhancing autophagy and the AMPK/mTOR signaling. Biomed. Pharmacother. 2018, 108, 1201–1207.
- Fu, D.; Yu, J.Y.; Connell, A.R.; Yang, S.; Hookham, M.B.; McLeese, R.; Lyons, T.J. Beneficial Effects of Berberine on Oxidized LDL-Induced Cytotoxicity to Human Retinal Müller Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3369–3379.
- Yu, J.Y.; Lyons, T.J. Modified Lipoproteins in Diabetic Retinopathy: A Local Action in the Retina. J. Clin. Exp. Ophthalmol. 2013, 4, 314.
- Goh, S.-Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152.
- Libby, P.; Nathan, D.M.; Abraham, K.; Brunzell, J.D.; Fradkin, J.E.; Haffner, S.M.; Hsueh, W.; Rewers, M.; Roberts, B.T.; Savage, P.J.; et al. National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus, Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 2005, 111, 3489–3493.
- Hao, M.H.; Li, S.-Y.; Sun, C.-K.; Xu, J.; Lin, Y.; Liu, K.-X.; Wang, L.; Li, C.-X.; Zhou, Q.; Du, J.-L.; et al. Amelioration effects of berberine on diabetic microendothelial injury model by the combination of high glucose and advanced glycation end products in vitro. Eur. J. Pharmacol. 2011, 654, 320–325.
- Wang, Y.; Huang, Y.; Lam, K.S.; Li, Y.; Wong, W.T.; Ye, H.; Lau, C.-W.; Vanhoutte, P.M.; Xu, A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc. Res. 2009, 82, 484–492.
- Liu, M.; Gao, L.; Zhang, N. Berberine reduces neuroglia activation and inflammation in streptozotocin-induced diabetic mice. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419866379.
- Kumaş, M.; Eşrefoğlu, M.; Karataş, E.; Duymaç, N.; Kanbay, S.; Ergün, I.S.; Üyüklü, M.; Koçyiğit, A. Investigation of dose-dependent effects of berberine against renal ischemia/reperfusion injury in experimental diabetic rats. Nefrologia 2019, 39, 411–423.
- Ding, B.; Geng, S.; Hou, X.; Ma, X.; Xu, H.; Yang, F.; Liu, K.; Liang, W.; Ma, G. Berberine reduces renal cell pyroptosis in golden hamsters with diabetic nephropathy through the Nrf2-NLRP3-Caspase-1-GSDMD pathway. Evid.-Based Complement. Altern. Med. 2021, 2021, 5545193.
- Chang, W.; Li, K.; Guan, F.; Yao, F.; Yu, Y.; Zhang, M.; Hatch, G.M.; Chen, L. Berberine pretreatment confers cardioprotection against ischemia–reperfusion injury in a rat model of type 2 diabetes. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 486–494.
- Xuan, W.-T.; Wang, H.; Zhou, P.; Ye, T.; Gao, H.-W.; Ye, S.; Wang, J.-H.; Chen, M.-L.; Song, H.; Wang, Y.; et al. Berberine ameliorates rats model of combined Alzheimer’s disease and type 2 diabetes mellitus via the suppression of endoplasmic reticulum stress. 3 Biotech 2020, 10, 359.
- Chu, X.; Zhou, Y.; Zhang, B.; Xue, B.; Zhao, Y. Berberine attenuates cerebral ischemia-reperfusion injury via activating PI3K-Akt signaling in a rat model of type 2 diabetes. Int. J. Clin. Exp. Med. 2017, 10, 16196–16202.
- Zhou, G.; Yan, M.; Guo, G.; Tong, N. Ameliorative effect of berberine on neonatally induced type 2 diabetic neuropathy via modulation of BDNF, IGF-1, PPAR-γ, and AMPK expressions. Dose Response 2019, 17, 1559325819862449.
- Wang, K.; Chen, Q.; Wu, N.; Li, Y.; Zhang, R.; Wang, J.; Gong, D.; Zou, X.; Liu, C.; Chen, J. Berberine ameliorates spatial learning memory impairment and modulates cholinergic anti-inflammatory pathway in diabetic rats. Front. Pharmacol. 2019, 10, 1003.
- Wang, S.; Ren, H.; Zhong, H.; Zhao, X.; Li, C.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; Yang, J.; et al. Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: A double blinded placebo controlled randomized study. Gut Microbes 2022, 14, 2003176.
- Kulkarni, S.K.; Dandiya, P.C.; Varandani, N.L. Pharmacological investigations of berberine sulphate. Jpn. J. Pharmacol. 1972, 22, 11–16.
- Lampe, D. Waste watch. Nat. Civ. Rev. 1992, 81, 192–194.
This entry is offline, you can click here to edit this entry!
