Plastics have recently become an indispensable part of everyone’s daily life due to their versatility, durability, light weight, and low production costs. The increasing production and use of plastics poses great environmental problems due to their incomplete utilization, a very long period of biodegradation, and a negative impact on living organisms. Decomposing plastics lead to the formation of microplastics, which accumulate in the environment and living organisms, becoming part of the food chain. The contamination of soils and water with poly(vinyl chloride) (PVC) seriously threatens ecosystems around the world. Their durability and low weight make microplastic particles easily transported through water or air, ending up in the soil.
- poly(vinyl chloride)
- PVC
- pollution
- aquatic environment
1. Introduction
2. PVC Characteristics
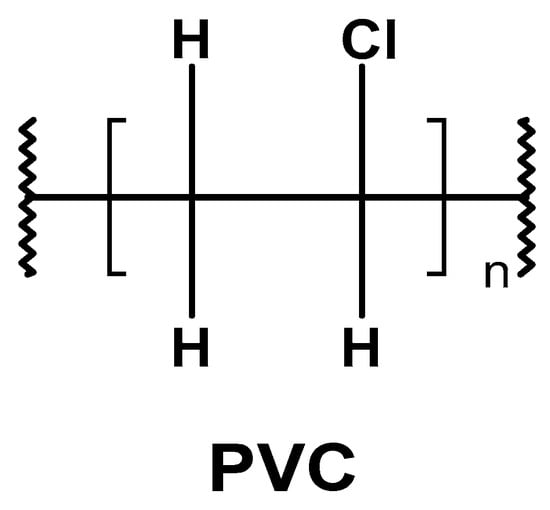
2.1. PVC’s Physical Properties
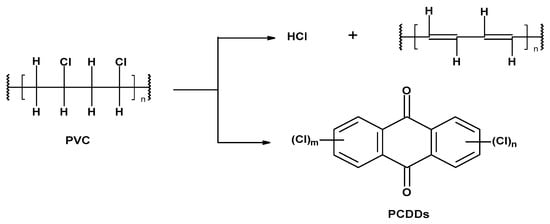
2.2. PVC’s Chemical Properties
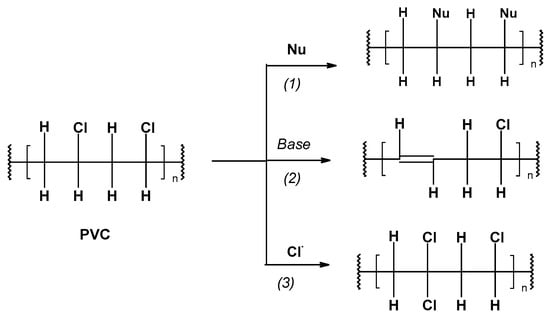
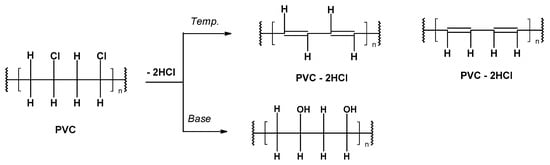
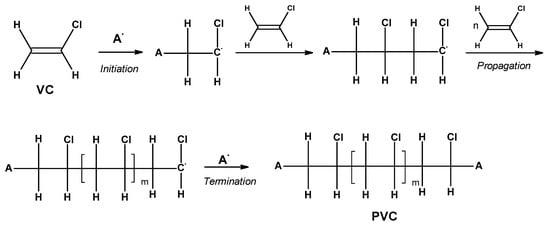
-
Preliminary (occurring under the influence of temperature, called thermal) under anaerobic conditions at the molecular or ionic level,
-
Secondary as a result of the increased temperature and oxygen action (thermo-oxidative degradation).
2.3. Biological Activity of Poly(vinyl chloride)
3. Disposal Methods of Poly(vinyl chloride) from the Environment
3.1. Recycling and Utilization of PVC
3.2. Biodegradation of PVC Waste
This entry is adapted from the peer-reviewed paper 10.3390/ma17010173
References
- De-la-Torre, G.E.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; Santillán, L. New plastic formations in the Anthropocene. Sci. Total Environ. 2021, 754, 14221–14226.
- Plastics Europe. Plastics—The Facts 2021. 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 2 October 2023).
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2020, 703, 134722.
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2023, 3, e1700782.
- Bouaicha, O.; Mimmo, T.; Tiziani, R.; Praeg, N.; Polidori, C.; Lucini, L.; Vigani, G.; Terzano, R.; Sanchez-Hernandez, J.C.; Illmer, P.; et al. Microplastics make their way into the soil and rhizosphere: A review of the ecological consequences. Rhizosphere 2022, 22, 100542.
- Xu, Y.; Xian, Z.-N.; Yue, W.; Yin, C.-F.; Zho, N.-Y. Degradation of polyvinyl chloride by a bacterial consortium enriched from the gut of Tenebrio molitor larvae. Chemosphere 2023, 318, 137944.
- Bermúdez, J.R.; Swarzenski, P.W. A microplastic size classification scheme aligned with universal plankton survey methods. MethodsX 2021, 8, 101516.
- Barili, S.; Bernetti, A.; Sannino, C.; Montegiove, N.; Calzoni, E.; Cesaretti, A.; Pinchuk, I.; Pezzolla, D.; Turchetti, B.; Buzzini, P.; et al. Impact of PVC microplastics on soil chemical and microbiological parameters. Environ. Res. 2023, 229, 115891.
- Available online: http://tts-polska.com/tts-polska2/informacje/polichlorek-winylu.html (accessed on 3 October 2023).
- Available online: https://www.globaldata.com/store/report/polyvinyl-chloride-market-analysis/ (accessed on 4 October 2023).
- Miliute-Plepiene, J.; Fråne, A.; Almasi, A.M. Overview of polyvinyl chloride (PVC) waste management practices in the Nordic countries. Clean. Eng. Technol. 2021, 4, 100246.
- Uropean Commission. The Use of PVC (Poly Vinyl Chloride) in the Contex of a Non-Toxic Environment. 2022. Available online: https://op.europa.eu/en/publication-detail/-/publication/e9e7684a-906b-11ec-b4e4-01aa75ed71a1 (accessed on 5 October 2023).
- Available online: http://archive.greenpeace.org/toxics/html/contenUpvc1.html (accessed on 5 October 2023).
- Available online: http://www.greenpeace.org/usa/en/campaigns/toxics/go-pvc-free/ (accessed on 6 October 2023).
- Available online: http://www.who.inUmediacentre/factsheets/fs225/en/ (accessed on 7 October 2023).
- Available online: http://www.greenpeace.org/usa/en/campaigns/!oxics/go-pvc-free/ (accessed on 7 October 2023).
- Available online: http://www.ens-newswire.com/ens/jan2011/2011-01-21-01.htmlhttps://www.google.com/search?client=firefox-b-d&q=PVC-Free+Future%3A+A+Review+of+Restrictions+and+PVC+free+Policies+Worldwide (accessed on 9 October 2023).
- Titow, W.V. (Ed.) PVC Polymers BT—PVC Plastics: Properties, Processing, and Applications; Springer: Dordrecht, The Netherlands, 1990; pp. 53–101.
- Fisher, I.; Schmitt, W.F.; Porth, H.C.; Allsopp, M.W.; Vianello, G. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2014.
- Gilbert, M.; Patrick, S. Poly(Vinyl Chloride). In Brydson’s Plastics Materials, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 329–388.
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314.
- Endo, K. Synthesis and structure of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2021–2054.
- Braun, D. PVC—Origin, growth, and future. J. Vinyl Addit. Technol. 2001, 7, 168–176.
- Braun, D. Poly(vinyl chloride) on the way from the 19th century to the 21st century. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 578–586.
- Saeki, Y.; Emura, T. Technical progresses for PVC production. Prog. Polym. Sci. 2002, 27, 2055–2131.
- Thornton, J. Environmental Impacts of Polyvinyl Chloride Building Materials. Healthy Buiding Network. 2002. Available online: https://www.google.com/search?client=firefox-b-d&q=Environmental+Impacts+of+Polyvinyl+Chloride+Building+Materials (accessed on 10 October 2023).
- Howard, M. Exploring the Global Polyvinyl Chloride (PVC) Market Size: Trends, Challenges, and Opportunities, Demand, Growth. 2030. Available online: https://Www.Zionmarketresearch.Com/Sample/Polyvinyl-Chloride-Pvc-Market (accessed on 11 October 2023).
- Moulay, S. Chemical modification of poly(vinyl chloride)—Still on the run. Prog. Polym. Sci. 2010, 35, 303–331.
- Skelly, P.W.; Li, L.; Braslau, R. Internal plasticization of PVC. Polym. Rev. 2022, 62, 485–528.
- Lieberzeit, P.; Bekchanov, D.; Mukhamedie, M. Polyvinyl chloride modifications, properties, and applications: Review. Polym. Adv. Technol. 2022, 33, 1809–1820.
- Babinsky, R. PVC additives: A global review. Plast. Addit. Compd. 2006, 8, 38–40.
- Mark, J.E. (Ed.) Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007.
- PPI TR-19. The Plastics Pipe Institute, Inc. TR-19. Chemical Resistance of Plastic Piping Materials. 28 April 2023. Available online: https://www.plasticpipe.org/ (accessed on 11 October 2023).
- Grause, G.; Hirahashi, S.; Toyoda, H.; Kameda, T.; Yoshioka, T. Solubility parameters for determining optimal solvents for separating PVC from PVC-coated PET fibers. J. Mater. Cycles Waste Manag. 2015, 19, 612–622.
- Kameda, T.; Fukuda, Y.; Grause, G.; Yoshioka, T. Chemical modification of rigid poly(vinyl chloride) by the substitution with nucleophiles. J. Appl. Polym. Sci. 2010, 116, 36–44.
- Kameda, T.; Ono, M.; Grause, G.; Mizoguchi, T.; Yoshioka, T. Chemical modification of poly(vinyl chloride) by nucleophilic substitution. Polym. Degrad. Stab. 2009, 94, 107–112.
- Bacaloglu, R.; Fisch, M. Reaction mechanism of poly(vinyl chloride) degradation. Molecular orbital calculations. J. Vinyl Addit. Technol. 1995, 1, 241–249.
- Ge, X.; Starnes, W.H. Chlorination of poly(vinyl chloride) model compounds in radical-complexing solvents. J. Vinyl Addit. Technol. 2016, 22, 405–409.
- Zakharyan, E.M.; Petrukhina, N.N.; Dzhabarov, E.G.; Maksimov, A.L. Pathways of Chemical Recycling of Polyvinyl Chloride. Part 2. Russ. J. Appl. Chem. 2020, 93, 1445–1490.
- Zakharyan, E.M.; Petrukhina, N.N.; Maksimov, A.L. Pathways of Chemical Recycling of Polyvinyl Chloride: Part 1. Russ. J. Appl. Chem. 2020, 93, 1271–1313.
- Lewandowski, K.; Skórczewska, K. A Brief Review of Poly(Vinyl Chloride) (PVC) Recycling. Polymers 2022, 14, 3035.
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423, 127238.
- Pospíšil, J.; Horák, Z.; Kruliš, Z.; Nešpůrek, S.; Kuroda, S. Degradation and aging of polymer blends I. Thermomechanical and thermal degradation. Polym. Degrad. Stab. 1999, 65, 405–414.
- Carroll, W.F., Jr.; Berger, T.C.; Borrelli, F.E.; Garrity, P.J.; Jacobs, R.A.; Ledvina, J.; Lewis, J.W.; McCreedy, R.L.; Smith, T.P.; Tuhovak, D.R.; et al. Characterization of emissions of dioxins and furans from ethylene dichloride, vinyl chloride monomer and polyvinyl chloride facilities in the United States. Consolidated report. Chemosphere 2001, 43, 689–700.
- Available online: https://oxoplast.com/stabilnosc-polichlorku-winylu/ (accessed on 12 October 2023).
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ. Toxicol. Pharmacol. 2019, 72, 103239.
- Chen, W.; Gong, Y.; McKie, M.; Almuhtaram, H.; Sun, J.; Barrett, H.; Yang, D.; Wu, M.; Andrews, R.C.; Peng, H. Defining the chemical additives driving in vitro toxicities of plastics. Environ. Sci. Technol. 2022, 56, 14627–14639.
- Yuan, Z.; Nag, R.; Cummins, E. Human health concerns regarding microplastics in the aquatic environment—From marine to food systems. Sci. Total Environ. 2022, 823, 153730.
- Wagoner, J.K. Toxicity of vinyl chloride and poly(vinyl chloride): A critical review. Environ. Health Perspect. 1983, 52, 61–66.
- Huang, C.-Y.; Huang, K.-L.; Cheng, T.-J.; Wang, J.-D.; Hsieh, L.-L. The GST T1 and CYP2E1 genotypes are possible factors causing vinyl chloride induced abnormal liver function. Archiv. Toxicol. 1997, 71, 482–488.
- Sass, J.B.; Castleman, B.; Wallinga, D. Vinyl Chloride: A Case study of data suppression and misrepresentation. Environ. Health Perspect. 2005, 113, 809–812.
- Kapp, R.W. Vinyl chloride. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 934–938.
- Mahadevan, G.; Valiyaveettil, S. Comparison of genotoxicity and cytotoxicity of polyvinyl chloride and poly(methyl methacrylate) nanoparticles on normal human lung cell lines. Chem. Res. Toxicol. 2021, 34, 1468–1480.
- Stock, V.; Laurisch, C.; Franke, J.; Dönmez, M.H.; Voss, L.; Böhmert, L.; Braeuning, A.; Sieg, H. Uptake and cellular effects of PE, PP, PET and PVC microplastic particles. Toxicol. In Vitro 2021, 70, 105021.
- Paul, M.B.; Fahrenson, C.; Givelet, L.; Herrmann, T.; Loeschner, K.; Böhmert, L.; Thünemann, A.F.; Braeuning, A.; Sieg, H. Beyond microplastics—Investigation on health impacts of submicron and nanoplastic particles after oral uptake in vitro. Microplastics Nanoplastics 2022, 2, 16.
- Guardiola, J.J.; Beier, J.I.; Falkner, K.C.; Wheeler, B.; McClain, C.J.; Cave, M. Occupational exposures at a polyvinyl chloride production facility are associated with significant changes to the plasma metabolome. Toxicol. Appl. Pharmacol. 2016, 313, 47–56.
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267.
- Oleru, U.G.; Onyekwere, C. Exposures to polyvinyl chloride, methyl ketone and other chemicals—The pulmonary and non-pulmonary effect. Int. Archiv. Occup. Environ. Health 1992, 63, 503–507.
- Xu, H.; Hoet, P.H.; Nemery, B. In vitro toxicity assessment of polyvinyl chloride particles and comparison of six cellular systems. J. Toxicol. Environ. Health A 2002, 65, 1141–1159.
- Zelko, I.N.; Taylor, B.S.; Das, T.P.; Watson, W.H.; Sithu, I.D.; Wahlang, B.; Malovichko, M.V.; Cave, M.C.; Srivastava, S. Effect of vinyl chloride exposure on cardiometabolic toxicity. Environ. Toxicol. 2022, 37, 245–255.
- Ju, P.; Zhang, Y.; Zheng, Y.; Gao, F.; Jiang, F.; Li, J.; Sun, C. Probing the toxic interactions between polyvinyl chloride microplastics and Human Serum Albumin by multispectroscopic techniques. Sci. Total Environ. 2020, 734, 139219.
- Ju, P.; Zhang, Y.; Ding, J.; Jiang, F.; Sun, C.; Jiang, F.; Sun, C. New insights into the toxic interactions of polyvinyl chloride microplastics with bovine serum albumin. Environ. Sci. Pollut. Res. 2021, 28, 5520–5531.
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Chronic exposure to polyvinyl chloride microplastics induces liver injury and gut microbiota dysbiosis based on the integration of liver transcriptome profiles and full-length 16S rRNA sequencing data. Sci. Total Environ. 2022, 839, 155984.
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl chloride microplastics induced gut barrier dysfunction, microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809.
- Richards, R.J.; Desai, R.; Hext, P.M.; Rose, F.A. Biological reactivity of PVC dust. Nature 1975, 5519, 664–665.
- Sharman, M.; Rose, M.; Parker, I.; Mercer, A.; Castle, L.; Gilbert, J.; Startin, J. Migration from plasticized films into foods. 1. Migration of di-(2-ethylhexyl)adipate from PVC films during home-use and microwave cooking. Food Addit. Contam. 1987, 4, 385–398.
- Sampson, J.; De Korte, D. Review DEHP-plasticised PVC: Relevance to blood services. Transfus. Med. 2011, 21, 73–83.
- Olkova, A. Toxicity of water after short-term contact with pvc materials depending on the temperature and components of the polymer composition. Ecol. Eng. Environ. Technol. 2021, 22, 119–125.
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic products leach chemicals that induce in vitrotoxicity under realistic use conditions. Environ. Sci. Technol. 2021, 55, 11814–11823.
- Fishbein, L. Toxicity of the components of poly(vinylchloride) polymers additives. Prog. Clin. Biol. Res. 1983, 141, 113–136.
- Beiras, R.; Verdejo, E.; Campoy-López, P.; Vidal-Liñán, L. Aquatic toxicity of chemically defined microplastics can be explained by functional additives. J. Hazard. Mater. 2021, 406, 124338.
- Yang, H.; Li, X.; Guo, M.; Cao, X.; Zheng, X.; Bao, D. UV-induced microplastics (MPs) aging leads to comprehensive toxicity. Mar. Pollut. Bull. 2023, 189, 114745.
- Li, W.; Wang, Z.; Li, W.; Li, Z. Impacts of microplastics addition on sediment environmental properties, enzymatic activities and bacterial diversity. Chemosphere 2022, 307, 135836.
- Shue, M.F.; Liou, J.J.; Tasi, J.L.; Tang, H.C.; Huang, W.J.; Liao, M.H. Cytotoxicity studies on combustion gas of polyvinyl chloride (PVC) resin. Aerosol Air Qual. Res. 2009, 9, 305–308.
- Sokolova, Y.; Gotlib, E.; Kozhevnikov, R.; Sokolova, A. Modification of PVC-compositions for linoleum. IOP Conf. Ser. Mater. Sci. Eng. 2018, 365, 032021.
- Gotlib, E.; Sadykova, D.; Vdovina, T.; Galeeva, L.; Sokolova, A. Evaluation of bactericidal properties of PVC-compositions for linoleum production. E3S Web Conf. 2019, 97, 02001.
- European Commission. Green Paper—Environmental Issues of PVC. 2000. Available online: https://eur-lex.europa.eu/legal-content/SL/TXT/?uri=CELEX:52000DC0469 (accessed on 16 October 2023).
- VinylPlus. Progress Report. Reporting on 2017 Activities. 2018. Available online: https://vinylplus.eu/wp-content/uploads/2021/06/VinylPlus-Progress-Report-2018-PbyP.pdf (accessed on 27 October 2018).
- Takeshita, T.; Kato, K.; Takahashi, K.K.; Sato, Y.; Nishi, S. Basic study on treatment of waste polyvinyl chloride plastics by hydrothermal decomposition in subcritical and supercritical regions. J. Supercrit. Fluids 2004, 31, 185–193.
- Lu, L.; Kumagai, S.; Kameda, T.; Luo, L.; Yoshioka, T. Degradation of PVC waste into a flexible polymer by chemical modification using DINP moieties. RSC Adv. 2019, 9, 28870–28875.
- Zhao, P.; Li, T.; Yan, W.; Yuan, L. Dechlorination of PVC wastes by hydrothermal treatment using alkaline additives. Environ. Technol. 2018, 39, 977–985.
- Xiu, F.-R.; Lu, Y.; Qi, Y. DEHP degradation and dechlorination of polyvinyl chloride waste in subcritical water with alkali and ethanol: A comparative study. Chemosphere 2020, 249, 126138.
- Xiu, F.-R.; Zhou, K.; Qi, Y. Co-treatment of PVC and used LCD panels in low-temperature subcritical water: Enhanced dechlorination and mechanism. Process Saf. Environ. Prot. 2021, 151, 10–19.
- Yuan, G.; Chen, D.; Yin, L.; Wang, Z.; Zhao, L.; Wang, J.Y. High efficiency chlorine removal from polyvinyl chloride (PVC) pyrolysis with a gas–liquid fluidized bed reactor. Waste Manag. 2014, 34, 1045–1050.
- Yang, R.; Zhao, Z.; Pu, Y.; Xiao, K.; Liu, R.; Cao, H.; Wang, Y.; Wang, X. Study of the photoaging process of polyvinyl chloride in different media with the electrical sensing zone method. Reg. Stud. Mar. Sci. 2023, 65, 103073.
- Yousif, E.; Hasan, A. Photostabilization of poly(vinyl chloride)—Still on the run. J. Taibah Univ. Sci. 2015, 9, 421–448.
- Decker, C. Photodegradation of PVC. In Degradation and Stabilisation of PVC; Owen, E.D., Ed.; Springer: Dordrecht, The Netherlands, 1984; pp. 81–136.
- Sil, D.; Chakrabarti, S. Photocatalytic degradation of PVC–ZnO composite film under tropical sunlight and artificial UV radiation: A comparative study. Sol. Energy 2010, 84, 476–485.
- Zhang, Y.; Sun, T.; Zhang, D.; Shi, Z.; Zhang, X.; Li, C.; Wang, L.; Song, J.; Lin, Q. Enhanced photodegradability of PVC plastics film by codoping nano-graphite and TiO2. Polym. Degrad. Stab. 2020, 181, 109332.
- Ali, M.; Perveen, Q.; Ahmad, B.; Javed, I.; Razi-Ul-Hussnain, R.; Andleeb, S.; Atique, N.; Ghumro, P.B.; Ahmed, S.; Hameed, A. Studies on Biodegradation of Cellulose Blended Polyvinyl Chloride Films. Int. J. Agric. Biol. 2009, 11, 577–580.
- Bahl, S.; Dolma, J.; Jyot Singh, J.; Sehgal, S. Biodegradation of plastics: A state of the art review. Mater. Today Proc. 2021, 39, 31–34.
- Alshehrei, F. Biodegradation of Synthetic and Natural Plastic by Microorganisms. J. Appl. Environ. Microbiol. 2017, 5, 8–19.
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Biodegradation of polyvinyl chloride plastic films by enriched anaerobic marine consortia. Mar. Environ. Res. 2020, 158, 104949.
- Sakhalkar, S.; Mishra, R.L. Screening and identification of soil fungi with potential of plastic degrading ability. Indian J. Appl. Res. 2013, 3, 62–64.
- Čolnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Degradation of Polyvinyl Chloride (PVC) Waste with Supercritical Water. Processes 2022, 10, 1940.
- Yin, F.; Zhuang, Q.; Chang, T.; Zhang, C.; Sun, H.; Sun, Q.; Wang, C.; Li, L. Study on pyrolysis characteristics and kinetics of mixed plastic waste. J. Mater. Cycles Waste Manag. 2021, 23, 1984–1994.
- Sadat-Shojai, M.; Bakhshandeh, G.-R. Recycling of PVC wastes. Polym. Degrad. Stab. 2011, 96, 404–415.
- Lu, L.; Li, W.; Cheng, Y.; Liu, M. Chemical recycling technologies for PVC waste and PVC-containing plastic waste: A review. Waste Manag. 2023, 166, 245–258.
- Baitz, M.; Kreißig, J.; Byrne, E.; Makishi, C.; Kupfer, T.; Frees, N.; Bey, N.; Hansen, M.S.; Hansen, A.; Bosch, T.; et al. Final Report: ”Life Cycle Assessment of PVC and of Principal Competing Materials”, Commissioned by the European Commission; European Commission: Luxembourg, 2004.
- Ali, M.F.; Siddiqui, M.N. Thermal and catalytic decomposition behavior of PVC mixed plastic waste with petroleum residue. J. Anal. Appl. Pyrolysis 2005, 74, 282–289.
- Jiang, X.; Zhu, B.; Zhu, M. An overview on the recycling of waste poly(vinyl chloride). Green Chem. 2023, 25, 6971–7025.
- Lu, J.; Ma, S.; Gao, J. Study on the Pressurized Hydrolysis Dechlorination of PVC. Energy Fuels 2002, 16, 1251–1255.
- Ma, D.; Liang, L.; Hu, E.; Chen, H.; Wang, D.; He, C.; Feng, Q. Dechlorination of polyvinyl chloride by hydrothermal treatment with cupric ion. Process Saf. Environ. Prot. 2021, 146, 108–117.
- Ammala, A.; Bateman, S.; Dean, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049.
- Amobonye, A.E.; Bhagwat, P.; Singh, S.; Pillai, S. Chapter 10—Biodegradability of Polyvinyl chloride. In Biodegradability of Conventional Plastics; Sarkar, A., Sharma, B., Shekhar, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 201–220.
- Ganesh, K.A.; Anjana, K.; Hinduja, M.; Sujitha, K.; Dharani, G. Review on plastic wastes in marine environment—Biodegradation and biotechnological solutions. Mar. Pollut. Bull. 2020, 150, 110733.
- Restrepo-Flórez, J.-M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene—A review. Int. Biodeterior. Biodegrad. 2014, 88, 83–90.
- Ho, B.T.; Roberts, T.K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Crit. Rev. Biotechnol. 2018, 38, 308–320.
- Zhang, Y.-T.; Wei, W.; Sun, J.; Xu, Q.; Ni, B.-J. Long-Term Effects of Polyvinyl Chloride Microplastics on Anaerobic Granular Sludge for Recovering Methane from Wastewater. Environ. Sci. Technol. 2020, 54, 9662–9671.
- Peng, B.-Y.; Chen, Z.; Chen, J.; Yu, H.; Zhou, X.; Criddle, C.S.; Wu, W.-M.; Zhang, Y. Biodegradation of Polyvinyl Chloride (PVC) in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Environ. Int. 2020, 145, 106106.
- Vivi, V.K.; Martins-Franchetti, S.M.; Attili-Angelis, D. Biodegradation of PCL and PVC: Chaetomium globosum (ATCC 16021) activity. Folia Microbiol. 2019, 64, 1–7.
- Klrbas, Z.; Güner, N.K.A. Biodegradation of Polyvinylchloride (PVC) by white rot fungi. Bull. Environ. Contam. Toxicol. 1999, 63, 335–342.
- Das, G.; Bordoloi, N.K.; Rai, S.K.; Mukherjee, A.K.; Karak, N. Biodegradable and biocompatible epoxidized vegetable oil modified thermostable poly(vinyl chloride): Thermal and performance characteristics post biodegradation with Pseudomonas aeruginosa and Achromobacter sp. J. Hazard. Mater. 2012, 209–210, 434–442.
- Ali, M.I.; Ahmed, S.; Robson, G.; Javed, I.; Ali, N.; Atiq, N.; Hameed, A. Isolation and molecular characterization of polyvinyl chloride (PVC) plastic degrading fungal isolates. J. Basic Microbiol. 2014, 54, 18–27.
- Khatoon, N.; Jamal, A.; Ali, M.I. Lignin peroxidase isoenzyme: A novel approach to biodegrade the toxic synthetic polymer waste. Environ. Technol. 2019, 40, 1366–1375.
- Zhang, Z.; Peng, H.; Yang, D.; Zhang, G.; Zhang, J.; Ju, F. Polyvinyl chloride degradation by a bacterium isolated from the gut of insect larvae. Nat. Commun. 2022, 13, 5360.
- Temporiti, M.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms 2022, 10, 1180.
- Rad, M.M.; Moghimi, H.; Azin, E. Biodegradation of thermo-oxidative pretreated low-density polyethylene (LDPE) and polyvinyl chloride (PVC) microplastics by Achromobacter denitrificans Ebl13. Mar. Pollut. Bull. 2022, 181, 113830.
- Tsochatzis, E.; Lopes, J.A.; Gika, H.; Theodoridis, G. Polystyrene biodegradation by Tenebrio molitor larvae: Identification of generated substances using a GC-MS untargeted screening method. Polymers 2021, 13, 17.
- Wertz, J.T.; Béchade, B. Chapter Three—Symbiont-mediated degradation of dietary carbon sources in social herbivorous insects. In Advances in Insect Physiology; Oliver, K.M., Russell, J.A., Eds.; Mechanisms Underlying Microbial Symbiosis; Academic Press: Cambridge, MA, USA, 2020; pp. 63–109.
- Bożek, M.; Hanus-Lorenz, B.; Rybak, J. The studies on waste biodegradation by Tenebrio molitor. E3S Web Conf. 2017, 17, 00011.
- Xu, H.; Dinsdale, D.; Nemery, B.; Hoet, P.H.M. Role of residual additives in the cytotoxicity and cytokine release caused by polyvinyl chloride particles in pulmonary cell cultures. Toxicol. Sci. 2003, 72, 92–102.
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. N. Biotechnol. 2019, 52, 35–41.
- Khandare, S.D.; Chaudhary, D.R.; Jha, B. Bioremediation of polyvinyl chloride (PVC) films by marine bacteria. Mar. Pollut. Bull. 2021, 169, 112566.
- Patil, R.; Bagde, U.S. Isolation of polyvinyl chloride degrading bacterial strains from environmental samples using enrichment culture technique. Afr. J. Biotechnol. 2012, 11, 7947–7956.
- Webb, J.S.; Nixon, M.; Eastwood, I.M.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungal Colonization and Biodeterioration of Plasticized Polyvinyl Chloride. Appl. Environ. Microbiol. 2000, 66, 3194–3200.
- Ali, M.I.; Ahmed, S.; Javed, I.; Ali, N.; Atiq, N.; Hameed, A.; Robson, G. Biodegradation of starch blended polyvinyl chloride films by isolated Phanerochaete chrysosporium PV1. Int. J. Environ. Sci. Technol. 2014, 11, 339–348.
- Saeed, S.; Iqbal, A.; Deeba, F. Biodegradation study of Polyethylene and PVC using naturally occurring plastic degrading microbes. Arch. Microbiol. 2022, 204, 497.
- Pardo-Rodríguez, M.L.; Zorro-Mateus, P.J.P. Biodegradation of polyvinyl chloride by Mucor sp. and Penicillium sp. isolated from soil. Rev. Investig. Desarro. Innov. 2021, 11, 387–400.
- Abdel-Naby, A.S.; Al-Ghamdi, A.A. Poly(vinyl chloride) blend with biodegradable cellulose acetate in presence of N-(phenyl amino) maleimides. Int. J. Biol. Macromol. 2014, 70, 124–130.
- Kaczmarek, H.; Bajer, K. Biodegradation of plasticized poly(vinyl chloride) containing cellulose. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 903–919.
- Danko, A.S.; Meizhong, L.; Bagwell, C.E.; Brigmon, R.L.; Freedman, D.L. Involvement of Linear Plasmids in Aerobic Biodegradation of Vinyl Chloride. Appl. Environ. Microbiol. 2004, 70, 6092–6097.
- Othman, A.R.; Hasan, H.A.; Muhamad, M.H.; ’Izzati Ismail, N.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073.
- Shimao, M.; Tamogami, T.; Kishida, S.; Harayama, S. The gene pvaB encodes oxidized polyvinyl alcohol hydrolase of Pseudomonas sp. strain VM15C and forms an operon with the polyvinyl alcohol dehydrogenase gene pvaAThe DDBJ accession number for the sequence reported in this paper is AB008494. Microbiology 2000, 146, 649–657.
- Yang, X.-G.; Wen, P.-P.; Yang, Y.-F.; Jia, P.-P.; Li, W.-G.; Pei, D.-S. Plastic biodegradation by in vitro environmental microorganisms and in vivo gut microorganisms of insects. Front. Microbiol. 2023, 13, 1001750.
- Sumathi, T.; Viswanath, B.; Lakshmi, A.S.; SaiGopal, D.V.R. Production of Laccase by Cochliobolus sp. isolated from plastic dumped soils and their ability to degrade low molecular weight PVC. Biochem. Res. Int. 2016, 2016, 9519527.
