Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Schizophrenia is a serious and debilitating neurodevelopmental disorder that typically occurs in early adulthood. DNA methylation, a critical epigenetic modification, contributes to alter gene expression without affecting the underlying genomic sequences; 5-methycytosine (5mC) and 5-hydroxylcytosine (5hmC) are two major forms of DNA methylation in mammals.
- schizophrenia
- epigenetic
- DNA methylation
- 5mC
- 5hmC
1. Introduction
Schizophrenia is a serious and debilitating neurodevelopmental disorder that typically occurs in early adulthood. Patients experience hallucinations, delusions, disorganization, and deterioration in cognitive and social functioning, such as amotivation and social withdrawal [1]. The average lifetime prevalence of schizophrenia is just under 1% [2]. Individuals from all ages with schizophrenia have high mortality and have their life expectancy reduced by approximately 20 years compared with the general population [3]. Alterations in cortical circuits have been reported to be involved in the pathogenesis of schizophrenia. The loss of dendritic spines in pyramidal cells results in reduced excitatory activity, while, at the same time, reduced excitatory input to GABAergic interneurons results in reduced inhibition in pyramidal cells. These changes are thought to lead to an abnormal functional network [4].
The heritability of schizophrenia is as high as 80% [5]. Genetic factors, including copy number variants [6] and de novo mutations [7], have been identified to be closely associated with the development of schizophrenia. Interestingly, studies of monozygotic twins have revealed the heterogeneity of schizophrenia, suggesting that non-genetic factors may be involved in the pathogenesis of schizophrenia. Environmental factors can induce enduring changes in gene expression through epigenetic mechanisms in neurodevelopment processes, aging, and neurodegenerative diseases [8]. DNA methylation, a critical epigenetic modification, contributes to alter gene expression without affecting the underlying genomic sequences; 5-methycytosine (5mC) and 5-hydroxylcytosine (5hmC) are two major forms of DNA methylation in mammals. DNA methyltransferases (DNMTs) transfer a methyl group to the 5-position of cytosine to form 5mC; the ten-eleven translocation (TET) dioxygenases catalyze the 5mC to 5hmC, 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) on DNA.
Altering the expression or activity of DNA methylation-related enzymes produce exacerbated phenotypes or therapeutic effects in different animal models. Cathrin Bayer et al. found that the depletion of DNMT1 increased the survival of mutant Huntington-transfected cells and accelerated perinuclear Huntington aggregation, suggesting that DNMT1-dependent degradation pathways mediate mutant Huntington-induced cytotoxicity [9]. Growing evidence suggests that epigenetic responses to environmental stimuli and plays an important role in schizophrenia [10]. By mapping thousands of 5mC sites in the prefrontal cortex of schizophrenia patients and healthy controls, differentially methylated regions (DMRs) at genes related to development and neurodifferentiation have been identified [11]. Disruptions of genome-wide 5mC have been observed in peripheral blood mononuclear cells (PBMCs) of schizophrenia patients, particularly in those experiencing their first episode of disease [12]. Despite inconsistent results from genome-wide association studies (GWAS), 108 replicable genomic loci have been identified in 2014 [6].
2. Altered 5mC Pattern in Schizophrenia
It is known that 5mC is the primary product of DNA methylation, which can influence various processes such as gene activity, individual development, and cancer progression [13][14]. DNMT1, DNMT3A, and DNMT3B possess catalytic activity and covalently add a methyl group from S-adenosylmethionine (SAM) onto the fifth carbon of the cytosine (C) pyrimidine ring, resulting in the formation of 5mC. While DNMT2 and DNMT3L contain conserved sequences of DNMT enzymes, they lack catalytic activity [15][16]. During late embryonic development and after maturation, genomic methylation patterns are established, and DNMT1 is responsible for maintaining the genomic methylation status. The DNMT1 is approximately one hundred times more active than DNMT3A and DNMT3B [17].
Abnormalities of 5mC have been widely reported in neurodegenerative diseases, such as Parkinson’s disease (PD) and Huntington’s disease (HD) [18][19][20]. Psychiatric disorders are characterized by impairment in cognition, emotion regulation, or behavior, and typically include bipolar disorder (BD) and schizophrenia. The pathogenesis of BD involves mitochondrial dysfunction, DNA oxidative damage, and DNA methylation changes. BD patients and their relatives exhibit genome-wide decreases in 5mC levels. After lithium treatment, 5mC levels remained unchanged in BD patients, whereas the levels were increased in their relatives, providing evidence of the significance of 5mC modification in BD pathology [21][22][23].
The overexpression of DNMT1 was considered to be one of the etiological factors of schizophrenia. Transcriptional dysregulation has been observed in embryonic stem cells overexpressing DNMT1. Approximately 50% of these genes have been implicated in schizophrenia, showing dysregulation independent of DNA methylation [24][25]. A novel GWAS in patients with schizophrenia identified schizophrenia-associated differential 5mC at 242 sites, of which mitotic arrest deficient 1-like 1 (MAD1L1) was robustly differentially methylated [26].
Alongside GWAS, a growing number of studies aim to identify the 5mC of candidate genes in patients with schizophrenia [27]. Specific 5mC differences have also been found in the gray and white matter of individuals with schizophrenia. These DMRs were identified within or near the Kruppel-like factor 9 (KLF9), sideroflexin 1 (SFXN1), Sprouty-related EVH1 domain-containing 2 (SPRED2), and AlS2 C-terminal-like (ALS2CL) genes [28]. Other key genes with dynamic 5mC were also involved in the pathogenesis of schizophrenia (Table 1).
Table 1. Association of 5mC with schizophrenia.
| DMRs | Tissues | Expression | 5mC | Phenotypes | References |
|---|---|---|---|---|---|
| KLF9 | Human cortical grey and white matter | ↓ | ↑ | rs11142387 near the KLF9 was significantly associated with psychiatric disease and poor memory function. | [28] |
| SFXN1 | Human cortical grey and white matter | ↓ | ↑ | The loss of SPRED2 leads to defective glycine and purine synthesis. | [28] |
| SPRED2 | Human cortical grey and white matter | ↓ | ↑ | The loss of SPRED2 leads to a phenotype resembling recessive Noonan syndrome. | [28] |
| ALS2CL | Human cortical grey and white matter | ↑ | ↓ | Mutations in ALS2CL may contribute to the development of schizophrenia. | [28] |
| RELN | Human peripheral blood | ↓ | ↑ | Single-allele and biallelic mutations in RELN can lead to neurodevelopmental disorders. The dysregulation of RELN expression has been observed in patients with schizophrenia and bipolar disorder. | [29][30] |
| BDNF | Human peripheral blood | ↓ | ↑ | BDNF activates the tyrosine kinase receptor B (TrkB), triggering various downstream signaling pathways. In patients with schizophrenia, there are alterations in BDNF signaling transduction. | [31] |
| SLC6A3 | Isohelix swab pack | ↓ | ↑ | SLC6A3 is associated with several neurological and psychiatric disorders, including ADHD, autism, cognitive impairments, movement disorders, and schizophrenia. | [32][33] |
| DTNBP1 | Human brain | ↓ | ↑ | The aberrant expression of DTNBP1B is associated with cognitive deficits in schizophrenia. | [34][35][36] |
| GAD1 | Human | ↓ | ↑ | The GAD1-knockout mouse model exhibits impairments in spatial memory and working memory. It shows reduced locomotor activity in new environments and a decreased preference for novel stimuli. | [37] |
| COMT | Human peripheral blood | ↑ | ↑ | The deletion of the COMT gene can lead to a range of complex complications, with psychiatric symptoms manifesting as schizophrenia and other mental disorders. | [38] |
↑: upregulation; ↓: downregulation.
Reelin protein is a signaling molecule that plays a crucial role in the nervous system. In humans, the dysregulation of Reelin protein is associated with various neurological disorders, including BD, autism, depression, and schizophrenia [39][40]. It is encoded by the RELN gene and was originally discovered in recessive mutant mice. The abnormal expression of this protein can lead to manifestations such as gait disturbances, ataxia, and tremors [39]. Reelin protein regulates the adhesion and migration of nerve cells by activating receptors on the cell surface, thereby promoting the proper positioning and layering of cerebral cortex neurons [39]. In humans, the dysregulation of Reelin protein is associated with various neurological disorders, including schizophrenia, BD, autism, depression, and more [39][40]. Reelin was found to be altered in various animal models of schizophrenia, suggesting its key role in the pathogenesis of schizophrenia [41]. Reelin levels were significantly reduced in schizophrenia patients, with specific gender differences [42]. The reduction of Reelin in schizophrenia was attributed to a significant increase in 5mC levels at the RELN promoter, with unmethylated Reelin expressed 25-fold higher than methylated Reelin [29][30]. Therefore, 5mC is an important factor in regulating RELN expression, and exploring the precise mechanism of the methylation of RELN may help elucidate the pathogenesis of schizophrenia.
Brain-derived neurotrophic factor (BDNF) regulates neurogenesis and synaptic plasticity as a biomarker for neuropsychiatric disorders [31]. such as bipolar disorder and major depressive disorder, and alterations in BDNF levels, the imbalance between pro-BDNF and m-BDNF, and defects in the BDNF signaling pathway have been observed [43]. When the BDNF Val66Met was knocked into mice, BDNF expression was significantly decreased, resulting in reduced neurogenesis and structural abnormalities in the hippocampus [44][45]. In patients with chronic schizophrenia, BDNF levels are closely related to memory, attention, information-processing speed, and cognitive impairment [46]. The 5mC of the BDNF gene could be regulated by environmental conditions and was believed to be involved in the pathogenesis of schizophrenia. Using bisulfite sequencing, no differences were found in BDNF 5mC status between schizophrenia patients and healthy controls, but there was a correlation between disease progression and 5mC [47]. An analysis of postmortem brains from schizophrenia patients revealed elevated levels of 5mC and 5hmC in the BDNF-regulatory regions in the frontal cortex and hippocampus, including the promoter regions, suggesting a strong association between BDNF methylation and schizophrenia [31]. Furthermore, schizophrenia patients with the same genotype and alleles exhibited different symptoms, which may be related to the differences in BDNF expression levels. Additionally, BDNF levels may differ in schizophrenia patients treated with different drugs [48].
The specific mechanisms underlying schizophrenia is still completely unclear, but classical theories believe that serotonin, glutamate, and dopamine networks are closely associated with the onset of schizophrenia [49]. Glutamate decarboxylase 1 (GAD1) is an important factor in the glutamate metabolism network; it plays a crucial role in conditions such as attention deficit/hyperactivity disorder (ADHD), heroin addiction, early infantile epilepsy, and developmental delay [50][51][52]. In patients with schizophrenia, 5mC levels in the promoter region of GAD1 were reduced, resulting in a significant increase in the overall expression of GAD1 [37][53]. The COMT gene encodes catechol-O-methyltransferase and is involved in the regulation of the dopamine metabolism network, it is involved in treatment-resistant depression, borderline personality disorder, post-traumatic stress disorder, and other mental disorders [54][55][56]. In patients with schizophrenia, the 5mC levels of the COMT gene are elevated, resulting in a significant increase in mRNA expression. The dysregulation of COMT expression has been demonstrated in the pathophysiology of schizophrenia [38][57][58] Dopamine transporter (DAT) is encoded by the Solute Carrier Family 6 Member 3 (SLC6A3) gene and is located on the neuronal cell membrane. It is responsible for the reuptake of dopamine from the synaptic cleft into neurons, thereby regulating the concentration and duration of the dopamine action in neurons. Therefore, it plays a role in emotional control, reward regulation, and other processes [59][60]. Moreover, 5mC levels in the DAT 5′-UTR of PD patients were different from healthy controls, and this methylation level may be related to different stages of the disease [61]. Cognitive impairment is one of the symptoms in schizophrenia patients. DAT availability in the striatum has been showed to be positively correlated with cognitive function in schizophrenia patients. Reduced DAT availability was primarily associated with increased 5mC levels [32][33]. These findings indicated that DNA methylation regulates key genes in the dopamine-network-involved pathological progress of schizophrenia.
Dystrobrevin-binding protein 1 (DTNBP1) is also highly associated with schizophrenia [34]. DTNBP1 is considered to be one of the key genes in the glutamatergic system; it also plays a role in depression and temporal lobe epilepsy [62][63]. It can interact with BDNF to regulate activity-dependent BDNF secretion, neural development, and inhibitory circuit function. Abnormalities in the DTNBP1 pathway may lead to schizophrenia [64][65]. In rodents, the reduced expression of DTNBP1 resulted in abnormal neuronal growth and morphology. Additionally, DTNBP1 plays a role in schizophrenia by regulating neurotransmission through the modulation of N-methyl-D-aspartate receptors (NMDAR) and dopamine D2 receptors (DRD2) [34]. It has been reported that female schizophrenia patients have higher 5mC levels at the upstream CpG sites of DTNBP1. This result was consistent with the observed downregulation of DTNBP1 mRNA levels in schizophrenia patients. Furthermore, investigations of saliva and postmortem brain samples from schizophrenia patients have revealed increased levels of 5mC in the DTNBP1 promoter, suggesting the potential role for 5mC on DTNBP1 in schizophrenia [35][36]. To sum up, the above related 5mC changes are summarized in Figure 1.
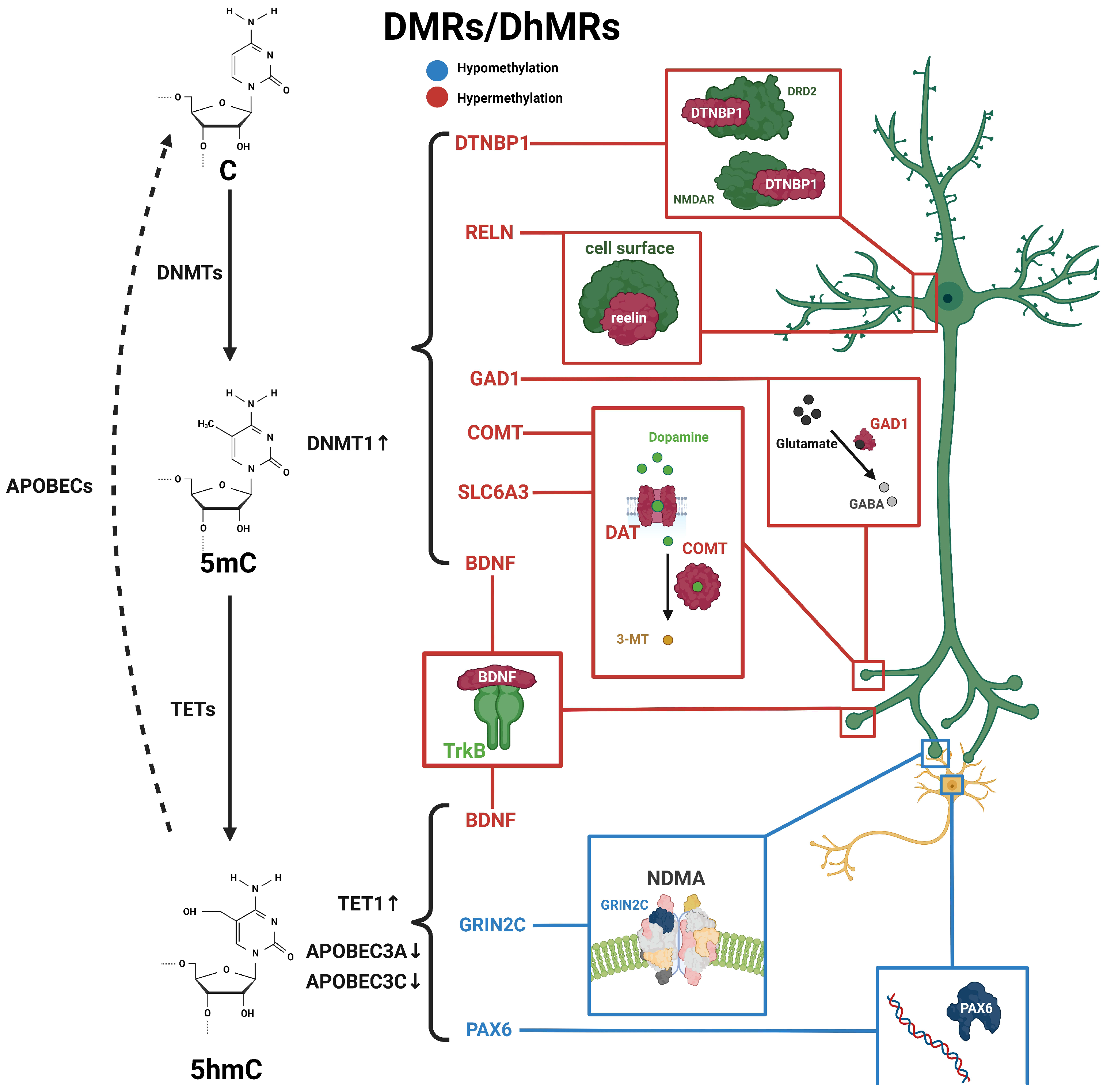
Figure 1. Schematic diagram of dynamic 5mC and 5hmC in neurons of schizophrenia patients. 5mC is catalyzed by DNMTs from cytosine and is then hydroxylated to 5hmC by TET enzymes, and further conversed into cytosine through the participation of apolipoprotein B mRNA-editing enzymes (APOBEC). Alterations in 5mC and 5hmC patterns have been identified in patients with schizophrenia. BDNF processes both 5mC and 5hmC dynamics. These distinctive patterns of 5mC and 5hmC are implicated in the pathogenesis and development of schizophrenia. Blue, hypomethylation; red, hypermethylation.
3. Altered 5hmC Pattern in Schizophrenia
In as early as 1952, 5hmC was discovered in bacteriophage DNA [66] and was first detected in mammalian genomes in 1972 [67]. As of 2009, the TET family has been identified to convert 5mC to 5hmC both in vivo and in vitro [68][69]. The TET protein family includes three members, TET1, TET2, and TET3, which have a conserved double-stranded β-helix domain (DSBH) at their C-terminus, which contains α-ketoglutarate (α-KG)- and Fe(II)-binding sites. Additionally, there is a cysteine-rich domain (CD) that contains a high concentration of cysteine residues. Together, these two domains form the core catalytic domain, which mediates the oxidation reaction of 5mC to 5hmC [70].
The distribution of 5hmC in animal tissues is widespread but variable, displaying certain tissue specificity [71][72][73]. It has been found, through 5hmC profiling in mammals, that 5hmC is mainly enriched in the central nervous system (CNS), with lower levels in peripheral tissues, which is in sharp contrast to the global distribution of 5mC [73][74]. In the brain, 5hmC exhibits region-specific distribution, with a unique distribution pattern in the cerebellum [75][76]. This suggests that 5hmC may play an important role in tissue-specific functions. Furthermore, 5hmC levels increase during neuronal differentiation and are associated with genes critical for neuronal function during neurodevelopment [77]. In addition, 5hmC is involved in processes of cell development and differentiation, the regulation of chromatin structure, and bone marrow regeneration [78][79][80]. In the process of DNA methylation regulation, the epigenetic plasticity of the CNS affects the expression of neuroactivity-dependent genes, organism learning, and memory [81][82].
It is well-known that 5mC is considered a stable heritable modification and represents a static process in epigenetic regulation. In fact, the dynamic balance between 5mC and 5hmC is an important condition for organism homeostasis, and imbalances in their ratio are implicated in the pathogenesis of many neurodegenerative diseases [78][79][83][84]. Abnormalities in 5hmC have been reported to be widespread in patients and animal models of AD and PD. At the same time, it was found that dynamic changes in 5hmC were positively correlated with aging [76][80].
Genome-wide DNA methylation changes may lead to genomic instability, and DNA methylation changes at promoter regions typically affect gene transcription. In schizophrenia, genome-wide 5hmC levels were elevated in male patients and reduced in female patients compared with healthy individuals [10], suggesting a potential association between the dysregulation of 5hmC levels and the development of schizophrenia. Many candidate genes in the peripheral blood or postmortem brain from schizophrenia patients have been identified with the differential methylation status associated with schizophrenia [11][85], including nitric oxide synthase 1 (NOS1), AKT serine/threonine kinase 1 (AKT1), DTNBP1, DNMT1, protein phosphatase 3 catalytic subunit gamma (PPP3CC), glutamic acid decarboxylase 67 (GAD67), and sex-determining region Y-box containing gene 10 (SOX10) [86][87]. These genes exhibit varying degrees of changes in 5mC or 5hmC (Figure 1 and Table 2).
Table 2. Association of 5hmC with schizophrenia.
| DhMRs | Tissues | Expression | 5hmC | Phenotypes | References |
|---|---|---|---|---|---|
| GABRB2 | Human, peripheral white blood cells | ↓ | ↑ | Gabrb2-knockout mice exhibit anxiety-like and depression-like behavioral changes, as well as alterations in social behavior, learning, and memory abilities. | [85] |
| GAD67 | Human, parietal cortex | ↓ | ↑ | GAD67-knockout mice exhibit emotional and auditory abnormalities, as well as anxiety-like behavior. | [87][88] |
| APOBEC3A/C | Human, parietal cortex and prefrontal cortex | ↓ | ↑ | The deletion of APOBEC3A has been associated with an increased susceptibility to early-onset breast cancer. | [87] |
| GADD45b | ↑ | ↑ | The knockdown of Gadd45b in the amygdala of neonatal rats leads to changes in social behavior during adolescence and a decrease in the expression of several genes associated with psychiatric disorders, including MeCP2, Reelin, and BDNF. | [89] | |
| BDNF IX | ↓ | ↑ | BDNF knockout mice exhibit chronic liver disease, specifically non-alcoholic fatty liver disease (NAFLD). | [89] | |
| GRIN2C | Monkey, cerebellum | ↓ | - | The knockdown of PAX6 in differentiating human limbal epithelial cells leads to a decrease in the expression of FABP5 and DSG1 proteins. | unpublished data |
| PAX6 | ↑ | - | unpublished data |
↑: upregulation; ↓: downregulation.
In the DNA demethylation pathway, apolipoprotein B mRNA-editing enzymes (APOBEC) are an important lipid carrier protein and participate in the conversion of 5hmC to 5fC and 5caC. TET1 expression was increased, while APOBEC3A and APOBEC3C expression was decreased in the frontal cortex of schizophrenia patients, suggesting that the disruption of the DNA methylation and demethylation pathways may contribute to elevated 5hmC levels in schizophrenia patients [87]. In addition, it has also been found that the increase in 5hmC occurring in the promoter region of the BDNF gene was associated with a reduced expression of BDNF in the frontal cortex of patients with schizophrenia [89]. Increased levels of 5mC and 5hmC in the BDNF promoter reduced the binding of GADD45b protein in the associated chromatin regulatory region and further reduced transcription levels in schizophrenia patients [89].
The dysregulation of methylation levels in schizophrenia-related genes plays a crucial role in the development of schizophrenia. However, research on 5hmC changes in schizophrenia remains limited. Further studies are needed to elucidate the detailed mechanisms by which 5hmC contributes to schizophrenia.
This entry is adapted from the peer-reviewed paper 10.3390/ijms25010636
References
- McCutcheon, R.A.; Marques, T.R.; Howes, O.D. Schizophrenia—An Overview. JAMA Psychiatry 2020, 77, 201–210.
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067.
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess Early Mortality in Schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448.
- Sheffield, J.M.; Barch, D.M. Cognition and Resting-State Functional Connectivity in Schizophrenia. Neurosci. Biobehav. Rev. 2016, 61, 108–120.
- Pepper, E.; Cardno, A.G. Genetics of Schizophrenia and Other Psychotic Disorders. Curr. Psychiatry Rev. 2014, 10, 133–142.
- Marshall, C.R.; Howrigan, D.P.; Merico, D.; Thiruvahindrapuram, B.; Wu, W.; Greer, D.S.; Antaki, D.; Shetty, A.; Holmans, P.A.; Pinto, D.; et al. Contribution of Copy Number Variants to Schizophrenia from a Genome-Wide Study of 41,321 Subjects. Nat. Genet. 2017, 49, 27–35.
- Zhou, Y.; Kaiser, T.; Monteiro, P.; Zhang, X.; Van der Goes, M.S.; Wang, D.; Barak, B.; Zeng, M.; Li, C.; Lu, C.; et al. Mice with Shank3 Mutations Associated with ASD and Schizophrenia Display Both Shared and Distinct Defects. Neuron 2016, 89, 147–162.
- Xie, J.; Xie, L.; Wei, H.; Li, X.-J.; Lin, L. Dynamic Regulation of DNA Methylation and Brain Functions. Biology 2023, 12, 152.
- Bayer, C.; Pitschelatow, G.; Hannemann, N.; Linde, J.; Reichard, J.; Pensold, D.; Zimmer-Bensch, G. DNA Methyltransferase 1 (DNMT1) Acts on Neurodegeneration by Modulating Proteostasis-Relevant Intracellular Processes. Int. J. Mol. Sci. 2020, 21, 5420.
- Jiang, T.; Zong, L.; Zhou, L.; Hou, Y.; Zhang, L.; Zheng, X.; Han, H.; Li, S.; Zhang, W.; Zhang, J.; et al. Variation in Global DNA Hydroxymethylation with Age Associated with Schizophrenia. Psychiatry Res. 2017, 257, 497–500.
- Jaffe, A.E.; Gao, Y.; Deep-Soboslay, A.; Tao, R.; Hyde, T.M.; Weinberger, D.R.; Kleinman, J.E. Mapping DNA Methylation across Development, Genotype and Schizophrenia in the Human Frontal Cortex. Nat. Neurosci. 2016, 19, 40–47.
- Ni, P.; Zhou, C.; Liang, S.; Jiang, Y.; Liu, D.; Shao, Z.; Noh, H.; Zhao, L.; Tian, Y.; Zhang, C.; et al. YBX1-Mediated DNA Methylation-Dependent SHANK3 Expression in PBMCs and Developing Cortical Interneurons in Schizophrenia. Adv. Sci. 2023, 10, e2300455.
- Greger, V.; Passarge, E.; Höpping, W.; Messmer, E.; Horsthemke, B. Epigenetic Changes May Contribute to the Formation and Spontaneous Regression of Retinoblastoma. Hum. Genet. 1989, 83, 155–158.
- Bunz, U.H.F. How Are Alkynes Scrambled? Science 2005, 308, 216–217.
- Lyko, F. The DNA Methyltransferase Family: A Versatile Toolkit for Epigenetic Regulation. Nat. Rev. Genet. 2018, 19, 81–92.
- Li, E.; Zhang, Y. DNA Methylation in Mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133.
- Mohan, K.N. DNMT1: Catalytic and Non-Catalytic Roles in Different Biological Processes. Epigenomics 2022, 14, 629–643.
- Zsindely, N.; Siági, F.; Bodai, L. DNA Methylation in Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 12736.
- Horvath, S.; Langfelder, P.; Kwak, S.; Aaronson, J.; Rosinski, J.; Vogt, T.F.; Eszes, M.; Faull, R.L.M.; Curtis, M.A.; Waldvogel, H.J.; et al. Huntington’s Disease Accelerates Epigenetic Aging of Human Brain and Disrupts DNA Methylation Levels. Aging 2016, 8, 1485–1512.
- Kaut, O.; Kuchelmeister, K.; Moehl, C.; Wüllner, U. 5-Methylcytosine and 5-Hydroxymethylcytosine in Brains of Patients with Multiple System Atrophy and Patients with Parkinson’s Disease. J. Chem. Neuroanat. 2019, 96, 41–48.
- Huzayyin, A.A.; Andreazza, A.C.; Turecki, G.; Cruceanu, C.; Rouleau, G.A.; Alda, M.; Young, L.T. Decreased Global Methylation in Patients with Bipolar Disorder Who Respond to Lithium. Int. J. Neuropsychopharmacol. 2014, 17, 561–569.
- Konradi, C.; Eaton, M.; MacDonald, M.L.; Walsh, J.; Benes, F.M.; Heckers, S. Molecular Evidence for Mitochondrial Dysfunction in Bipolar Disorder. Arch. Gen. Psychiatry 2004, 61, 300–308.
- Mustak, M.S.; Hegde, M.L.; Dinesh, A.; Britton, G.B.; Berrocal, R.; Subba Rao, K.; Shamasundar, N.M.; Rao, K.S.J.; Sathyanarayana Rao, T.S. Evidence of Altered DNA Integrity in the Brain Regions of Suicidal Victims of Bipolar Depression. Indian. J. Psychiatry 2010, 52, 220–228.
- Saxena, S.; Choudhury, S.; Maroju, P.A.; Anne, A.; Kumar, L.; Mohan, K.N. Dysregulation of Schizophrenia-Associated Genes and Genome-Wide Hypomethylation in Neurons Overexpressing DNMT1. Epigenomics 2021, 13, 1539–1555.
- Saxena, S.; Maroju, P.A.; Choudhury, S.; Anne, A.; Mohan, K.N. Analysis of Transcript Levels of a Few Schizophrenia Candidate Genes in Neurons from a Transgenic Mouse Embryonic Stem Cell Model Overexpressing DNMT1. Gene 2020, 757, 144934.
- McKinney, B.C.; McClain, L.L.; Hensler, C.M.; Wei, Y.; Klei, L.; Lewis, D.A.; Devlin, B.; Wang, J.; Ding, Y.; Sweet, R.A. Schizophrenia-Associated Differential DNA Methylation in Brain Is Distributed across the Genome and Annotated to MAD1L1, a Locus at Which DNA Methylation and Transcription Phenotypes Share Genetic Variation with Schizophrenia Risk. Transl. Psychiatry 2022, 12, 340.
- Hoffmann, A.; Sportelli, V.; Ziller, M.; Spengler, D. Epigenomics of Major Depressive Disorders and Schizophrenia: Early Life Decides. Int. J. Mol. Sci. 2017, 18, 1711.
- Berdenis van Berlekom, A.; Notman, N.; Sneeboer, M.A.; Snijders, G.J.; Houtepen, L.C.; Nispeling, D.M.; He, Y.; Dracheva, S.; Hol, E.M.; Kahn, R.S.; et al. DNA Methylation Differences in Cortical Grey and White Matter in Schizophrenia. Epigenomics 2021, 13, 1157–1169.
- Alfimova, M.V.; Kondratiev, N.V.; Golov, A.K.; Golimbet, V.E. Methylation of the Reelin Gene Promoter in Peripheral Blood and Its Relationship with the Cognitive Function of Schizophrenia Patients. Mol. Biol. 2018, 52, 676–685.
- Tamura, Y.; Kunugi, H.; Ohashi, J.; Hohjoh, H. The Possible Association between Epigenetic Aberration in DNA Methylation in RELN and Psychiatric Disorders. Mol. Psychiatry 2007, 12, 519.
- Fu, X.; Wang, J.; Du, J.; Sun, J.; Baranova, A.; Zhang, F. BDNF Gene’s Role in Schizophrenia: From Risk Allele to Methylation Implications. Front. Psychiatry 2020, 11, 564277.
- Chung, S.J.; Yoo, H.S.; Oh, J.S.; Kim, J.S.; Ye, B.S.; Sohn, Y.H.; Lee, P.H. Effect of Striatal Dopamine Depletion on Cognition in de Novo Parkinson’s Disease. Park. Relat. Disord. 2018, 51, 43–48.
- Cimino, S.; Cerniglia, L.; Ballarotto, G.; Marzilli, E.; Pascale, E.; D’Addario, C.; Adriani, W.; Tambelli, R. DNA Methylation at the DAT Promoter and Risk for Psychopathology: Intergenerational Transmission between School-Age Youths and Their Parents in a Community Sample. Front. Psychiatry 2017, 8, 303.
- Cheah, S.-Y.; Lawford, B.R.; Young, R.M.; Morris, C.P.; Voisey, J. Dysbindin (DTNBP1) Variants Are Associated with Hallucinations in Schizophrenia. Eur. Psychiatry 2015, 30, 486–491.
- Abdolmaleky, H.M.; Cheng, K.; Russo, A.; Smith, C.L.; Faraone, S.V.; Wilcox, M.; Shafa, R.; Glatt, S.J.; Nguyen, G.; Ponte, J.F.; et al. Hypermethylation of the Reelin (RELN) Promoter in the Brain of Schizophrenic Patients: A Preliminary Report. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 134B, 60–66.
- Abdolmaleky, H.M.; Pajouhanfar, S.; Faghankhani, M.; Joghataei, M.T.; Mostafavi, A.; Thiagalingam, S. Antipsychotic Drugs Attenuate Aberrant DNA Methylation of DTNBP1 (Dysbindin) Promoter in Saliva and Post-Mortem Brain of Patients with Schizophrenia and Psychotic Bipolar Disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015, 168, 687–696.
- Huang, H.-S.; Akbarian, S. GAD1 mRNA Expression and DNA Methylation in Prefrontal Cortex of Subjects with Schizophrenia. PLoS ONE 2007, 2, e809.
- Li, Z.; He, Y.; Han, H.; Zhou, Y.; Ma, X.; Wang, D.; Zhou, J.; Ren, H.; Yuan, L.; Tang, J.; et al. COMT, 5-HTR2A, and SLC6A4 mRNA Expressions in First-Episode Antipsychotic-Naïve Schizophrenia and Association With Treatment Outcomes. Front. Psychiatry 2018, 9, 577.
- Jossin, Y. Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation. Biomolecules 2020, 10, 964.
- Niu, S.; Yabut, O.; D’Arcangelo, G. The Reelin Signaling Pathway Promotes Dendritic Spine Development in Hippocampal Neurons. J. Neurosci. 2008, 28, 10339–10348.
- Sánchez-Hidalgo, A.C.; Martín-Cuevas, C.; Crespo-Facorro, B.; Garrido-Torres, N. Reelin Alterations, Behavioral Phenotypes, and Brain Anomalies in Schizophrenia: A Systematic Review of Insights From Rodent Models. Front. Neuroanat. 2022, 16, 844737.
- Bai, W.; Niu, Y.; Yu, X.; Yi, J.; Zhen, Q.; Kou, C. Decreased Serum Levels of Reelin in Patients with Schizophrenia. Asian J. Psychiatry 2020, 49, 101995.
- Shkundin, A.; Halaris, A. Associations of BDNF/BDNF-AS SNPs with Depression, Schizophrenia, and Bipolar Disorder. J. Pers. Med. 2023, 13, 1395.
- Chen, Z.-Y.; Jing, D.; Bath, K.G.; Ieraci, A.; Khan, T.; Siao, C.-J.; Herrera, D.G.; Toth, M.; Yang, C.; McEwen, B.S.; et al. Genetic Variant BDNF (Val66Met) Polymorphism Alters Anxiety-Related Behavior. Science 2006, 314, 140–143.
- Bath, K.G.; Jing, D.Q.; Dincheva, I.; Neeb, C.C.; Pattwell, S.S.; Chao, M.V.; Lee, F.S.; Ninan, I. BDNF Val66Met Impairs Fluoxetine-Induced Enhancement of Adult Hippocampus Plasticity. Neuropsychopharmacology 2012, 37, 1297–1304.
- Nieto, R.R.; Carrasco, A.; Corral, S.; Castillo, R.; Gaspar, P.A.; Bustamante, M.L.; Silva, H. BDNF as a Biomarker of Cognition in Schizophrenia/Psychosis: An Updated Review. Front. Psychiatry 2021, 12, 662407.
- Çöpoğlu, Ü.S.; Igci, M.; Bozgeyik, E.; Kokaçya, M.H.; İğci, Y.Z.; Dokuyucu, R.; Ari, M.; Savaş, H.A. DNA Methylation of BDNF Gene in Schizophrenia. Med. Sci. Monit. 2016, 22, 397–402.
- Huang, T.L. Effects of Antipsychotics on the BDNF in Schizophrenia. Curr. Med. Chem. 2013, 20, 345–350.
- Stahl, S.M. Beyond the Dopamine Hypothesis of Schizophrenia to Three Neural Networks of Psychosis: Dopamine, Serotonin, and Glutamate. CNS Spectr. 2018, 23, 187–191.
- Neuray, C.; Maroofian, R.; Scala, M.; Sultan, T.; Pai, G.S.; Mojarrad, M.; Khashab, H.E.; deHoll, L.; Yue, W.; Alsaif, H.S.; et al. Early-Infantile Onset Epilepsy and Developmental Delay Caused by Bi-Allelic GAD1 Variants. Brain 2020, 143, 2388–2397.
- Shi, Y.; Li, Y.; Zhang, J.; Xiao, Y.; Yan, P.; Zhu, Y. GAD1 but Not GAD2 Polymorphisms Are Associated with Heroin Addiction Phenotypes. Neurosci. Lett. 2020, 717, 134704.
- Kessi, M.; Duan, H.; Xiong, J.; Chen, B.; He, F.; Yang, L.; Ma, Y.; Bamgbade, O.A.; Peng, J.; Yin, F. Attention-Deficit/Hyperactive Disorder Updates. Front. Mol. Neurosci. 2022, 15, 925049.
- Tao, R.; Davis, K.N.; Li, C.; Shin, J.H.; Gao, Y.; Jaffe, A.E.; Gondré-Lewis, M.C.; Weinberger, D.R.; Kleinman, J.E.; Hyde, T.M. GAD1 Alternative Transcripts and DNA Methylation in Human Prefrontal Cortex and Hippocampus in Brain Development, Schizophrenia. Mol. Psychiatry 2018, 23, 1496–1505.
- Wang, Y.; Li, S.; Niu, L.; Ma, Y.; Qiu, Y.; Li, S.; Guobule, N.; Cao, H.; Li, J. Polymorphisms of COMT and CREB1 Are Associated with Treatment-Resistant Depression in a Chinese Han Population. J. Neural Transm. 2022, 129, 85–93.
- Su, M.; Song, Y. The Association between COMT Val158Met Polymorphism and the Post-Traumatic Stress Disorder Risk: A Meta-Analysis. Neuropsychobiology 2022, 81, 156–170.
- Thomas, M.; Banet, N.; Wallisch, A.; Glowacz, K.; Becker-Sadzio, J.; Gundel, F.; Nieratschker, V. Differential COMT DNA Methylation in Patients with Borderline Personality Disorder: Genotype Matters. Eur. Neuropsychopharmacol. 2019, 29, 1295–1300.
- Martínez-Jauand, M.; Sitges, C.; Rodríguez, V.; Picornell, A.; Ramon, M.; Buskila, D.; Montoya, P. Pain Sensitivity in Fibromyalgia Is Associated with Catechol-O-Methyltransferase (COMT) Gene. Eur. J. Pain 2013, 17, 16–27.
- Sesack, S.R.; Hawrylak, V.A.; Matus, C.; Guido, M.A.; Levey, A.I. Dopamine Axon Varicosities in the Prelimbic Division of the Rat Prefrontal Cortex Exhibit Sparse Immunoreactivity for the Dopamine Transporter. J. Neurosci. 1998, 18, 2697–2708.
- Marshall, V.; Grosset, D. Role of Dopamine Transporter Imaging in Routine Clinical Practice. Mov. Disord. 2003, 18, 1415–1423.
- Booth, T.C.; Nathan, M.; Waldman, A.D.; Quigley, A.-M.; Schapira, A.H.; Buscombe, J. The Role of Functional Dopamine-Transporter SPECT Imaging in Parkinsonian Syndromes, Part 1. AJNR Am. J. Neuroradiol. 2015, 36, 229–235.
- Rubino, A.; D’Addario, C.; Di Bartolomeo, M.; Michele Salamone, E.; Locuratolo, N.; Fattapposta, F.; Vanacore, N.; Pascale, E. DNA Methylation of the 5′-UTR DAT 1 Gene in Parkinson’s Disease Patients. Acta Neurol. Scand. 2020, 142, 275–280.
- Tao, H.; Zhou, X.; Chen, J.; Zhou, H.; Huang, L.; Cai, Y.; Fu, J.; Liu, Z.; Chen, Y.; Sun, C.; et al. Genetic Effects of the Schizophrenia-Related Gene DTNBP1 in Temporal Lobe Epilepsy. Front. Genet. 2021, 12, 553974.
- Domschke, K.; Lawford, B.; Young, R.; Voisey, J.; Morris, C.P.; Roehrs, T.; Hohoff, C.; Birosova, E.; Arolt, V.; Baune, B.T. Dysbindin (DTNBP1)—A Role in Psychotic Depression? J. Psychiatr. Res. 2011, 45, 588–595.
- Yang, Y.; Zhang, L.; Guo, D.; Zhang, L.; Yu, H.; Liu, Q.; Su, X.; Shao, M.; Song, M.; Zhang, Y.; et al. Association of DTNBP1 With Schizophrenia: Findings From Two Independent Samples of Han Chinese Population. Front. Psychiatry 2020, 11, 446.
- Jun, R.; Zhang, W.; Beacher, N.J.; Zhang, Y.; Li, Y.; Lin, D.-T. Dysbindin-1, BDNF, and GABAergic Transmission in Schizophrenia. Front. Psychiatry 2022, 13, 876749.
- Wyatt, G.R.; Cohen, S.S. A New Pyrimidine Base from Bacteriophage Nucleic Acids. Nature 1952, 170, 1072–1073.
- Penn, N.W.; Suwalski, R.; O’Riley, C.; Bojanowski, K.; Yura, R. The Presence of 5-Hydroxymethylcytosine in Animal Deoxyribonucleic Acid. Biochem. J. 1972, 126, 781–790.
- Kriaucionis, S.; Heintz, N. The Nuclear DNA Base 5-Hydroxymethylcytosine Is Present in Purkinje Neurons and the Brain. Science 2009, 324, 929–930.
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine in Mammalian DNA by MLL Partner TET1. Science 2009, 324, 930–935.
- Iyer, L.M.; Tahiliani, M.; Rao, A.; Aravind, L. Prediction of Novel Families of Enzymes Involved in Oxidative and Other Complex Modifications of Bases in Nucleic Acids. Cell Cycle 2009, 8, 1698–1710.
- Coppieters, N.; Dieriks, B.V.; Lill, C.; Faull, R.L.M.; Curtis, M.A.; Dragunow, M. Global Changes in DNA Methylation and Hydroxymethylation in Alzheimer’s Disease Human Brain. Neurobiol. Aging 2014, 35, 1334–1344.
- Li, W.; Liu, M. Distribution of 5-Hydroxymethylcytosine in Different Human Tissues. J. Nucleic Acids 2011, 2011, 870726.
- Globisch, D.; Münzel, M.; Müller, M.; Michalakis, S.; Wagner, M.; Koch, S.; Brückl, T.; Biel, M.; Carell, T. Tissue Distribution of 5-Hydroxymethylcytosine and Search for Active Demethylation Intermediates. PLoS ONE 2010, 5, e15367.
- Song, C.-X.; Szulwach, K.E.; Fu, Y.; Dai, Q.; Yi, C.; Li, X.; Li, Y.; Chen, C.-H.; Zhang, W.; Jian, X.; et al. Selective Chemical Labeling Reveals the Genome-Wide Distribution of 5-Hydroxymethylcytosine. Nat. Biotechnol. 2011, 29, 68–72.
- Santiago, M.; Antunes, C.; Guedes, M.; Sousa, N.; Marques, C.J. TET Enzymes and DNA Hydroxymethylation in Neural Development and Function—How Critical Are They? Genomics 2014, 104, 334–340.
- Xu, Y.; Zhong, L.; Wei, H.; Li, Y.; Xie, J.; Xie, L.; Chen, X.; Guo, X.; Yin, P.; Li, S.; et al. Brain Region- and Age-Dependent 5-Hydroxymethylcytosine Activity in the Non-Human Primate. Front. Aging Neurosci. 2022, 14, 934224.
- Hahn, M.A.; Qiu, R.; Wu, X.; Li, A.X.; Zhang, H.; Wang, J.; Jui, J.; Jin, S.-G.; Jiang, Y.; Pfeifer, G.P.; et al. Dynamics of 5-Hydroxymethylcytosine and Chromatin Marks in Mammalian Neurogenesis. Cell Rep. 2013, 3, 291–300.
- Haffner, M.C.; Chaux, A.; Meeker, A.K.; Esopi, D.M.; Gerber, J.; Pellakuru, L.G.; Toubaji, A.; Argani, P.; Iacobuzio-Donahue, C.; Nelson, W.G.; et al. Global 5-Hydroxymethylcytosine Content Is Significantly Reduced in Tissue Stem/Progenitor Cell Compartments and in Human Cancers. Oncotarget 2011, 2, 627–637.
- Mellén, M.; Ayata, P.; Dewell, S.; Kriaucionis, S.; Heintz, N. MeCP2 Binds to 5hmC Enriched within Active Genes and Accessible Chromatin in the Nervous System. Cell 2012, 151, 1417–1430.
- Szulwach, K.E.; Li, X.; Li, Y.; Song, C.-X.; Wu, H.; Dai, Q.; Irier, H.; Upadhyay, A.K.; Gearing, M.; Levey, A.I.; et al. 5-hmC-Mediated Epigenetic Dynamics during Postnatal Neurodevelopment and Aging. Nat. Neurosci. 2011, 14, 1607–1616.
- Chen, W.G.; Chang, Q.; Lin, Y.; Meissner, A.; West, A.E.; Griffith, E.C.; Jaenisch, R.; Greenberg, M.E. Derepression of BDNF Transcription Involves Calcium-Dependent Phosphorylation of MeCP2. Science 2003, 302, 885–889.
- Day, J.J.; Sweatt, J.D. DNA Methylation and Memory Formation. Nat. Neurosci. 2010, 13, 1319–1323.
- Villar-Menéndez, I.; Blanch, M.; Tyebji, S.; Pereira-Veiga, T.; Albasanz, J.L.; Martín, M.; Ferrer, I.; Pérez-Navarro, E.; Barrachina, M. Increased 5-Methylcytosine and Decreased 5-Hydroxymethylcytosine Levels Are Associated with Reduced Striatal A2AR Levels in Huntington’s Disease. Neuromol. Med. 2013, 15, 295–309.
- Chen, Y.; Ozturk, N.C.; Zhou, F.C. DNA Methylation Program in Developing Hippocampus and Its Alteration by Alcohol. PLoS ONE 2013, 8, e60503.
- Zong, L.; Zhou, L.; Hou, Y.; Zhang, L.; Jiang, W.; Zhang, W.; Wang, L.; Luo, X.; Wang, S.; Deng, C.; et al. Genetic and Epigenetic Regulation on the Transcription of GABRB2: Genotype-Dependent Hydroxymethylation and Methylation Alterations in Schizophrenia. J. Psychiatr. Res. 2017, 88, 9–17.
- Wockner, L.F.; Noble, E.P.; Lawford, B.R.; Young, R.M.; Morris, C.P.; Whitehall, V.L.J.; Voisey, J. Genome-Wide DNA Methylation Analysis of Human Brain Tissue from Schizophrenia Patients. Transl. Psychiatry 2014, 4, e339.
- Dong, E.; Gavin, D.P.; Chen, Y.; Davis, J. Upregulation of TET1 and Downregulation of APOBEC3A and APOBEC3C in the Parietal Cortex of Psychotic Patients. Transl. Psychiatry 2012, 2, e159.
- Melka, M.G.; Castellani, C.A.; Laufer, B.I.; Rajakumar, R.N.; O’Reilly, R.; Singh, S.M. Olanzapine Induced DNA Methylation Changes Support the Dopamine Hypothesis of Psychosis. J. Mol. Psychiatry 2013, 1, 19.
- Gavin, D.P.; Sharma, R.P.; Chase, K.A.; Matrisciano, F.; Dong, E.; Guidotti, A. Growth Arrest and DNA-Damage-Inducible, Beta (GADD45b)-Mediated DNA Demethylation in Major Psychosis. Neuropsychopharmacology 2012, 37, 531–542.
This entry is offline, you can click here to edit this entry!
