Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Microbiology
Influenza virus infections occur in people and animals worldwide and cause variable disease outcomes depending on the species affected and strain of the virus. Influenza viruses can be transmitted between animals, persons, or from animals to humans and can cause severe disease pathology or death.
- influenza virus
- IAV
- IBV
- pathology
1. Introduction
The family Orthomyxoviridae encompasses four genera of influenza viruses: Alphainfluenzavirus (influenza A virus, IAV), Betainfluenzavirus (influenza B virus, IBV), Gammainfluenzavirus (influenza C virus, ICV), and Deltainfluenzavirus (influenza D virus, IDV) [1]. Influenza virus infections occur in people and animals worldwide and cause variable disease outcomes depending on the species affected and strain of virus. IAV and IAB infect humans and are responsible for seasonal influenza (flu) epidemics that result in 3–5 million severe illnesses and 290,000–650,000 deaths yearly worldwide [2]. Global flu pandemics, due to the introduction of new, antigenically distinct IAV strains in immunologically naïve populations, occur sporadically and cause increased morbidity and mortality [3]. IAV has a broad host range, infecting aquatic birds, domestic poultry, pigs, dogs, horses, bats, and people. Aquatic birds are considered the reservoir species and likely source of pandemic IAV in humans [4]. In contrast, IBV and ICV are primarily human pathogens, although IBV has been isolated from seals [5] and ICV from cattle [6], pigs [7], and dogs [8]. Infection with ICV is less common and mainly affects children [9]. IDV is a newly emerging virus detected in pigs [10] and cattle [11] with potential zoonotic risk [12].
Orthomyxoviruses are enveloped, segmented, single-stranded, negative-sense RNA viruses. There are either 8 (IAV and IBV) or 7 (ICV and IDV) genome segments (Figure 1). The envelope of IAV and IBV is studded with the transmembrane glycoproteins hemagglutinin (HA) and neuraminidase (NA), which are responsible for attachment/penetration and virion release, respectively. IAVs are classified into subtypes based on their HA and NA molecules, of which there are 18 (HA) and 11 (NA) currently identified. All but two subtypes of IAV are found in aquatic birds while only H1N1 and H3N2 currently circulate in humans [13]. HA and NA are immunodominant epitopes with HA serving as the major target of neutralizing antibodies [14]. Random point mutations in HA and NA, due to the error-prone RNA polymerase, cause slow and gradual antigenic changes (antigenic drift) whereas genetic reassortment via exchange of gene segments results in new, antigenically distinct strains of virus (antigenic shift). These strategies allow the virus to evade host immune responses and develop resistance to antivirals. The possibility of zoonotic transmission and emergence of new pathogenic influenza strains of pandemic potential poses a significant public health threat.
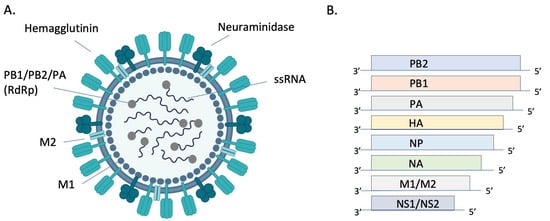
Figure 1. Structure and genomic organization of influenza A virus. The virion (A) consists of 8 negative sense ssRNA segments complexed with the nucleoprotein (NP, not pictured) to form the helical ribonucleoprotein (RNP). Each segment is associated with an RNA-dependent RNA polymerase (RdRP) complex composed of basic protein 1 (PB1), basic protein 2 (PB2), and the acidic protein (PA). Envelope-associated proteins include hemagglutinin (HA), neuraminidase (NA), matrix protein (M1), and the integral membrane protein (M2). Genomic segments 1–7 encode for one of these structural proteins and the 8th segment encodes for nonstructural proteins (NS) (B). Image (A) created with BioRender.com.
In addition to the expected annual flu burden, pandemic influenza viruses have emerged every 10–40 years due to the reassortment of human IAVs with gene segments of avian and/or swine origin [15]. These novel viruses result in increased morbidity and mortality due to lack of preexisting immunity in human populations [16]. The 1918 H1N1 “Spanish flu” was the deadliest pandemic in history, causing over 40 million deaths worldwide [17]. In addition to the human influenza viruses causing seasonal and pandemic disease, people are sporadically infected with highly pathogenic avian influenza (HPAI) viruses of the H5 or H7 subtype, resulting in approximately 1000 deaths to date [18] and mortality rates of up to 60% [19].
Influenza viruses are transmitted by inhalation of droplets and/or aerosols and by direct contact with an infected individual or contaminated surface [20]. Prevention strategies include nonpharmaceutical interventions such as hand washing, use of hand sanitizer [21,22], social distancing, cough and sneeze hygiene, and cleaning of potentially contaminated surfaces [3]. Vaccination remains the most effective way of preventing disease. Efficacy of inactivated and live attenuated vaccines typically varies between 40–60% [23] depending on the age and health status of an individual, virulence of the season’s major strains of virus, and how well that season’s vaccine matches circulating strains [3]. Antivirals play an important role in combating disease, although drug resistance is a major challenge. Continued research efforts to develop universal influenza vaccines and novel antivirals are essential to combat this highly infectious and potentially fatal disease.
2. Influenza Pathology
Influenza viruses enter host cells by binding of HA to sialic acid residues on glycoproteins and glycolipids. Binding affinity for specific sialic acid residues is an important determinant of host range, tissue tropism, and the potential for cross-species transmission (recently reviewed in ref [39]). In short, human influenza viruses preferentially bind to sialic acids with an α2,6 linkage to galactose (SAα2,6Gal) whereas avian influenza viruses preferentially bind to α2,3 linkages (SAα2,3Gal) [40]. In people, SAα2,6Gal is found predominantly in the upper airway and SAα2,3Gal mainly in the lower airway [41]. In contrast, birds express SAα2,3Gal predominantly in the upper airway and intestinal tract [42]. Thus, mutations that allow avian influenza viruses to bind to SAα2,6Gal in the upper respiratory tract of people are likely required for efficient human-to-human transmission via respiratory droplets and aerosols.
Influenza viruses replicate in the nucleus of respiratory and intestinal epithelial cells. Replication peaks at 48 h after infection and virus is shed for approximately 6–8 days [43]. Severity of disease is associated with viral replication in the lower respiratory tract [25]. While symptoms result from a combination of virus-mediated damage to epithelial cells and host immune responses (immunopathology), it is generally accepted that immunopathology plays the largest role in tissue damage [44]. Infected epithelial cells and innate immune cells release pro-inflammatory cytokines and chemokines that are important to control infection, but also lead to bystander damage to epithelial and endothelial cells. Excess neutrophil recruitment [45,46] and inflammatory cytokines such as IL-6, IL-8, IL-10, TNFα, CXCL10, IL-2R, GCSF, MCP1, and MIP1α are associated with disease severity and poor outcomes [35,47,48,49].
Because lung specimens are usually collected during autopsy, pathology is only documented in fatal cases of human IAV infection. Characteristic findings of viral pneumonia are similar between pandemic and non-pandemic years [25,32,34,50,51,52,53,54] so are described together. Grossly, the trachea and bronchi are hemorrhagic and often filled with blood-tinged, foamy fluid. The lungs are dark red and edematous, reflecting the underlying hemorrhagic bronchopneumonia that is frequently complicated by secondary bacterial infection [32,34,53]. Histologically, the trachea and bronchi have epithelial necrosis and desquamation in addition to submucosal edema, congestion, and hemorrhage. In the lower airways, there is necrotizing bronchiolitis and alveolitis, interstitial mononuclear inflammation, interstitial and alveolar edema, thrombi, hyaline membranes, and type II pneumocyte hyperplasia. These changes are consistent with the exudative phase of diffuse alveolar damage (DAD), which is the histologic hallmark of acute respiratory distress syndrome (ARDS) [55]. With time, epithelial regeneration, interstitial fibrosis, and bronchiolitis obliterans may develop. Secondary bacterial pneumonia consisting of overwhelming neutrophilic inflammation, extensive necrosis, and hemorrhage can obscure the underlying viral effects [25]. Hemophagocytosis is a prominent feature of some cases of fatal disease and is thought to be mediated by hypercytokinemia [33,51]. Hemophagocytosis was present in the lymph nodes of 18 of 36 (53%) pediatric patients dying of non-pandemic influenza [52] and in 25 of 41 (61%) patients dying of 2009 pH1N1 [33]. The clinical significance of hemophagocytosis in these infections is unclear.
While overall histologic findings are similar in fatal cases from pandemic and non-pandemic years (Table 1), antigen distribution appears to differ. In a study of 47 pediatric patients dying of seasonal influenza pneumonia from 2003–2004, antigen was detected primarily in the bronchial epithelial cells and mucous glands of the trachea, bronchi, and large bronchioles [52]. In contrast, antigen was mainly present in type I and II alveolar pneumocytes in patients dying of 2009 pandemic H1N1 (pH1N1) [33]. These findings likely reflect differences in tissue tropism based on receptor location and the variable course of disease at the time of sampling.
As the case numbers are significantly lower compared with seasonal and pandemic H1N1, there are fewer reports on the pathology of fatal avian H5N1 infection despite a case fatality rate of 56% [56]. Diffuse alveolar damage is the main histologic feature and most cases also display mild lymphocytic interstitial pneumonia, alveolar histiocytosis, hemorrhage, and type II pneumocyte hyperplasia [51,57,58,59,60,61,62,63,64]. Depending on the time course of disease, DAD may be exudative or organizing and fibrotic [62,63]. Extrapulmonary lesions including lymphoid depletion, hepatic necrosis, and acute tubular necrosis, are frequently reported [51,58,60,61,65]. Viral RNA can be detected outside of the respiratory tract in the spleen, liver, intestines [62,63,64], and cerebrospinal fluid [66] although extrapulmonary antigen is only rarely documented [61], suggesting that systemic spread may not be the culprit of multiorgan failure. In the respiratory tract, viral antigen and RNA are found most commonly in type I and II pneumocytes although they can also be present in macrophages, sloughed epithelial cells, non-ciliated and ciliated bronchiolar epithelium, and tracheal epithelium [57,58,60,61,62,63]. The predominant viral distribution in pneumocytes is consistent with the lower airway distribution of SAα2,3Gal, the receptor for avian influenza viruses [41]. Hemophagocytosis is a frequent finding in the lungs, lymph nodes, and spleen [51,57,58,60,61,63,64]. In severe cases, reactive hemophagocytic syndrome, consisting of pancytopenia, abnormal clotting times, and reduced liver function, is the most prominent finding [51,60]. A combination of high viral loads and an intense cytokine response appear central to the pathogenicity of H5N1 in people [57,67,68,69].
Table 1. Summary of clinical and pathologic findings in human and laboratory animal influenza infections.
| Common Animal Strain/Species | Virus Strain | Clinical Signs | Microscopic Pathology | References | |
|---|---|---|---|---|---|
| Humans | N/A | Seasonal IAV | Varying degrees of fever, non-productive cough, dyspnea, coryza, fatigue, and myalgia (classic flu symptoms); vomiting and diarrhea in severe cases | Necrotizing tracheobronchitis and bronchointerstitial pneumonia with thrombi, edema, hemorrhage, hyaline membranes, and type II pneumocyte hyperplasia (diffuse alveolar damage) | [25,26,50] |
| 2009 pH1N1 | Mild to severe flu symptoms | Same as seasonal IAV | [30,34,50,52] | ||
| 1918 H1N1 | Mild to severe flu symptoms | Same as seasonal IAV | [17,25,53] | ||
| HPAI H5N1 and H7N9 | Mild to severe flu symptoms with history of contact with live poultry | Same as seasonal IAV Extrapulmonary necrotic lesions are common and hemophagocytosis may be the most prominent lesion |
[19,35,36,37,50,51,70,71] | ||
| Mouse | BALB/c C57BL/6 DBA/2J A/J |
PR8 | Dyspnea, ruffled fur, weight loss, and anorexia | Interstitial pneumonia, suppurative bronchiolitis and alveolitis, hyaline membranes, and alveolar edema | [72,73,74,75] |
| BALB/c C57BL/6 |
2009 pH1N1 | Variable weight loss (dose and strain dependent) | Histiocytic to neutrophilic bronchitis, bronchiolitis and alveolitis with varying epithelial necrosis (strain dependent) | [76,77,78,79] | |
| BALB/c | 1918 H1N1 | Weight loss and death | Interstitial pneumonia, suppurative bronchiolitis and alveolitis, hyaline membranes, and alveolar edema | [76,80,81] | |
| BALB/c DBA/2J |
HPAI H5N1 | Dyspnea, ruffled fur, weight loss, and anorexia | Interstitial pneumonia, suppurative bronchiolitis and alveolitis, hyaline membranes, and alveolar edema Encephalitis and myocardial necrosis |
[81,82,83,84,85,86,87,88] | |
| C57BL/6 BALB/c |
H7N9 | Weight loss, ruffled fur, hunching | Bronchiolitis, patchy interstitial pneumonia, and varying amounts of bronchiolar and alveolar epithelial necrosis | [89,90,91,92] | |
| BALB/c | LPAI | Variable weight loss, ruffled fur, and hunching (strain dependent) | Necrotizing bronchitis and bronchiolitis with peribronchial pneumonia | [93,94,95,96,97] | |
| Hamster | Golden Syrian hamster | Seasonal IAV | Mild weight loss and temperature changes | None or mild necrotizing rhinitis and bronchopneumonia with perivascular cuffing | [98,99,100,101,102] |
| 2009 pH1N1 | Mild weight loss | Necrotizing rhinitis, bronchiolitis, perivasculitis, edema, and mild interstitial pneumonia | [101,103,104,105] | ||
| HPAI H5N1 | Not reported | Intranasal route: Bronchiolitis and bronchopneumonia Intragastric route: Interstitial pneumonia |
[106] | ||
| Ferret | Mustela putorius furo | Seasonal IAV | Asymptomatic or mild lethargy with sneezing, nasal discharge, and mild weight loss | Conventional intranasal model: Rhinitis and mild bronchiolitis and pneumoniaHigh dose intratracheal model: Moderate rhinitis and severe necrotizing bronchointerstitial pneumonia with edema | [107,108,109,110,111,112] |
| 2009 pH1N1 | Lethargy, anorexia, dyspnea, and elevated body temperature | Necrotizing rhinotracheitis, bronchitis, and bronchiolitis with varying degrees of interstitial pneumonia and diffuse alveolar damage | [77,110,111,112] | ||
| 1918 H1N1 | Weight loss, sneezing, dyspnea, lethargy, and death | Necrotizing rhinitis, bronchiolitis and bronchointerstitial pneumonia with edema | [113,114,115] | ||
| HPAI H5N1 | Lethargy, anorexia, dyspnea, nasal discharge, sneezing, weight loss, elevated body temperature, diarrhea, and neurologic signs (ataxia, torticollis, and hind limb paresis) | Severe necrotizing bronchointerstitial pneumonia with diffuse alveolar damage Meningoencephalitis |
[111,116,117,118,119] | ||
| LPAI | Transiently elevated body temperature and weight loss; occasional dyspnea and lethargy | Suppurative rhinitis | [91,120,121] | ||
| NHP | Cynomolgus macaques (most common), rhesus macaques, and common marmosets | Seasonal IAV | Asymptomatic or mild lethargy | Mild bronchointerstitial pneumonia and peribronchiolitis | [122,123] |
| 2009 pH1N1 | Asymptomatic or mildly elevated body temperature and lethargy; tachypnea, dyspnea, and nasal discharge reported for some strains of virus | Suppurative rhinitis in mild cases; necrotizing bronchopneumonia with edema and hyaline membranes in severe cases (strain dependent) | [124,125,126,127,128,129] | ||
| 1918 H1N1 | Anorexia, lethargy, cough, nasal discharge, and tachypnea | Necrotizing bronchointerstitial pneumonia with hemorrhage, edema, and hyaline membranes | [123,130,131] | ||
| HPAI H5N1 | Anorexia, lethargy, cough, tachypnea, elevated body temperature, diarrhea, and thrombocytopenia | Necrotizing bronchointerstitial pneumonia with hemorrhage, edema, hyaline membranes, and type II pneumocyte hyperplasia Lymphoid necrosis and renal tubular necrosis |
[130,132,133,134] |
This entry is adapted from the peer-reviewed paper 10.3390/pathogens13010035
This entry is offline, you can click here to edit this entry!
