An efficient carbon dioxide reduction reaction (CO2RR), which reduces CO2 to low-carbon fuels and high-value chemicals, is a promising approach for realizing the goal of carbon neutrality, for which effective but low-cost catalysts are critically important. Many inorganic perovskite-based materials with tunable chemical compositions have been applied in the electrochemical CO2RR, which exhibited advanced catalytic performance.
- perovskite
- synthesis
- CO2 electroreduction reaction
1. Introduction
|
Electrolyte Conditions |
Reactions |
E0/(V vs. RHE) |
Products |
|---|---|---|---|
|
/ |
2H + + 2e− → H2 |
0 |
hydrogen evolution reaction (HER) |
|
/ |
xCO2 + nH + + ne− → product + yH2O |
/ |
CO2RR |
|
Alkaline (pH: 8~14) |
CO2 + 2H + + 2e− → H + + HCOO– |
−0.12 |
formate |
|
CO2 + 2H + + 2e− → CO (g) + H2O |
−0.10 |
carbon monoxide |
|
|
CO2 + 6H + + 6e → CH3OH (aq) + H2O |
0.03 |
methanol |
|
|
CO2 + 4H + + 4e− → C (s) + 2H2O |
0.21 |
carbon |
|
|
CO2 + 8H + + 8e− → CH4 (g) + 2H2O |
0.17 |
methane |
|
|
2CO2 + 8H + + 8e− → H + + CH3COO– + 2H2O |
0.11 |
acetate |
|
|
2CO2 + 10H + + 10e− → CH3CHO (aq) + 3H2O |
0.06 |
acetaldehyde |
|
|
2CO2 + 12H + + 12e− → C2H5OH (aq) + 3H2O |
0.09 |
ethanol |
|
|
2CO2 + 2H + + 12e− → C2H4 (g) + 4H2O |
0.08 |
ethylene |
|
|
2CO2 + 14H + + 14e− → C2H6 (g) + 4H2O |
0.14 |
ethane |
|
|
3CO2 + 18H + + 18e− → C3H7OH (aq) + 5H2O |
0.10 |
propanol |
|
|
Acidic (pH: 0~7) |
CO2 + 2H + + 2e− → HCOOH (aq) |
−0.12 |
formic acid |
|
2CO2 + 8H + + 8e− → CH3COOH (aq) + 2H2O |
0.11 |
acetic acid |
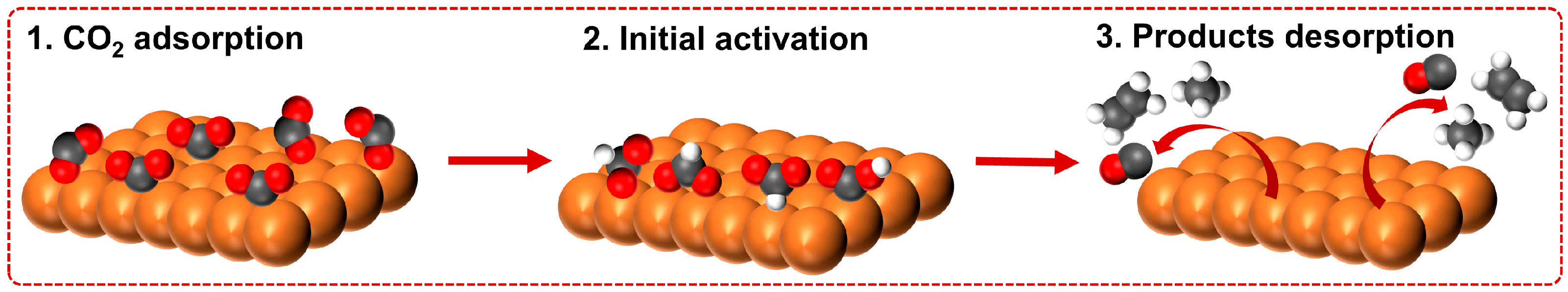
2. Fundamentals of CO2RR
3. Fundamentals of Perovskite Oxides
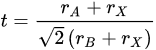
4. Fabrication Methods of Perovskite Materials
This entry is adapted from the peer-reviewed paper 10.3390/molecules28248154
References
- Shen, M.; Kong, F.; Tong, L.; Luo, Y.; Yin, S.; Liu, C.; Zhang, P.; Wang, L.; Chu, P.K.; Ding, Y. Carbon capture and storage (CCS): Development path based on carbon neutrality and economic policy. Carbon Neutrality 2022, 1, 37.
- Zheng, Y.; Ma, M.; Shao, H. Recent advances in efficient and scalable solar hydrogen production through water splitting. Carbon Neutrality 2023, 2, 23.
- Agency, I.E. CO2 Emissions in 2022; International Energy Agency: Paris, France, 2023.
- Agency, I.E. Global Energy Review: CO2 Emissions in 2021; International Energy Agency: Paris, France, 2021.
- Bai, X.F.; Chen, W.; Wang, B.Y.; Feng, G.H.; Wei, W.; Jiao, Z.; Sun, Y.H. Recent progress on electrochemical reduction of carbon dioxide. Acta Phys.-Chim. Sin. 2017, 33, 2388–2403.
- Van Vuuren, D.P.; Stehfest, E.; Gernaat, D.E.H.J.; van den Berg, M.; Bijl, D.L.; de Boer, H.S.; Daioglou, V.; Doelman, J.C.; Edelenbosch, O.Y.; Harmsen, M.; et al. Alternative pathways to the 1.5 °C target reduce the need for negative emission technologies. Nat. Clim. Chang. 2018, 8, 391–397.
- Handoko, A.D.; Wei, F.; Jenndy; Yeo, B.S.; Seh, Z.W. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat. Catal. 2018, 1, 922–934.
- Birdja, Y.Y.; Pérez-Gallent, E.; Figueiredo, M.C.; Göttle, A.J.; Calle-Vallejo, F.; Koper, M.T.M. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 2019, 4, 732–745.
- Yan, Y.; Ke, L.; Ding, Y.; Zhang, Y.; Rui, K.; Lin, H.; Zhu, J. Recent advances in Cu-based catalysts for electroreduction of carbon dioxide. Mater. Chem. Front. 2021, 5, 2668–2683.
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.Y.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and perspectives of electrochemical CO reduction on copper in aqueous electrolyte. Chem. Rev. 2019, 119, 7610–7672.
- Woldu, A.R.; Huang, Z.; Zhao, P.; Hu, L.; Astruc, D. Electrochemical CO2 reduction (CO2RR) to multi-carbon products over copper-based catalysts. Coord. Chem. Rev. 2022, 454, 214340.
- Huang, J.; Buonsanti, R. Colloidal nanocrystals as heterogeneous catalysts for electrochemical CO2 conversion. Chem. Mater. 2019, 31, 13–25.
- Zhu, P.; Wang, H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 2021, 4, 943–951.
- Yan, T.; Chen, X.; Kumari, L.; Lin, J.; Li, M.; Fan, Q.; Chi, H.; Meyer, T.J.; Zhang, S.; Ma, X. Multiscale CO2 electrocatalysis to C2+ products: Reaction mechanisms, catalyst design, and device fabrication. Chem. Rev. 2023, 123, 10530–10583.
- Xiao, J.; Liu, L.; Zhang, D.; De Marco, N.; Lee, J.W.; Lin, O.; Chen, Q.; Yang, Y. The emergence of the mixed perovskites and their applications as solar cells. Adv. Energy Mater. 2017, 7, 1700491.
- Retuerto, M.; Calle-Vallejo, F.; Pascual, L.; Lumbeeck, G.; Fernandez-Diaz, M.T.; Croft, M.; Gopalakrishnan, J.; Peña, M.A.; Hadermann, J.; Greenblatt, M.; et al. La1.5Sr0.5NiMn0.5Ru0.5O6 double perovskite with enhanced ORR/OER bifunctional catalytic activity. ACS Appl. Mater. Interfaces 2019, 11, 21454–21464.
- Zhu, Y.; Zhou, W.; Zhong, Y.; Bu, Y.; Chen, X.; Zhong, Q.; Liu, M.; Shao, Z. A perovskite nanorod as bifunctional electrocatalyst for overall water splitting. Adv. Energy Mater. 2017, 7, 1602122.
- Sun, Y.; Li, R.; Chen, X.; Wu, J.; Xie, Y.; Wang, X.; Ma, K.; Wang, L.; Zhang, Z.; Liao, Q.; et al. A-site management prompts the dynamic reconstructed active phase of perovskite oxide OER catalysts. Adv. Energy Mater. 2021, 11, 2003755.
- Xu, X.; Chen, Y.; Zhou, W.; Zhu, Z.; Su, C.; Liu, M.; Shao, Z. A perovskite electrocatalyst for efficient hydrogen evolution reaction. Adv. Mater. 2016, 28, 6442–6448.
- Li, D.; Zhang, D.; Lim, K.-S.; Hu, Y.; Rong, Y.; Mei, A.; Park, N.-G.; Han, H. A review on scaling up perovskite solar cells. Adv. Funct. Mater. 2021, 31, 2008621.
- Tilley, R.J. Perovskites: Structure-Property Relationships; John Wiley & Sons: Hoboken, NJ, USA, 2016.
- Xu, X.M.; Pan, Y.L.; Zhong, Y.J.; Ran, R.; Shao, Z.P. Ruddlesden-Popper perovskites in electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565.
- Yukta; Parikh, N.; Chavan, R.D.; Yadav, P.; Nazeeruddin, M.K.; Satapathi, S. Highly efficient and stable 2D Dion Jacobson/3D perovskite heterojunction solar cells. ACS Appl. Mater. Interfaces 2022, 14, 29744–29753.
- Kendall, K.R.; Navas, C.; Thomas, J.K.; zur Loye, H.-C. Recent developments in oxide Ion conductors: Aurivillius phases. Chem. Mater. 1996, 8, 642–649.
- Li, L.; Zhao, Z.; Hu, C.; Yang, P.; Yuan, X.; Wang, Y.; Zhang, L.; Moskaleva, L.; Gong, J. Tuning oxygen vacancies of oxides to promote electrocatalytic reduction of carbon dioxide. ACS Energy Lett. 2020, 5, 552–558.
- Lee, D.; Lee, H.N. Controlling oxygen mobility in Ruddlesden-Popper oxides. Materials 2017, 10, 368.
- Toda, K.; Kameo, Y.; Kurita, S.; Sato, M. Crystal structure determination and ionic conductivity of layered perovskite compounds NaLnTiO4 (Ln = rare earth). J. Alloys Compd. 1996, 234, 19–25.
- May, K.J.; Carlton, C.E.; Stoerzinger, K.A.; Risch, M.; Suntivich, J.; Lee, Y.-L.; Grimaud, A.; Shao-Horn, Y. Influence of oxygen evolution during water oxidation on the surface of perovskite oxide catalysts. J. Phys. Chem. Lett. 2012, 3, 3264–3270.
- Xu, X.; Su, C.; Shao, Z. Fundamental understanding and application of Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite in energy storage and conversion: Past, present, and future. Energy Fuels 2021, 35, 13585–13609.
- He, J.; Xu, X.; Li, M.; Zhou, S.; Zhou, W. Recent advances in perovskite oxides for non-enzymatic electrochemical sensors: A review. Anal. Chim. Acta 2023, 1251, 341007.
- Peng, X.; Feng, S.; Lai, S.; Liu, Z.; Gao, J.; Javanbakht, M.; Gao, B. Structural engineering of rare-earth-based perovskite electrocatalysts for advanced oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 39470–39485.
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2018, 2, 1800071.
- Han, X.; Hu, Y.; Yang, J.; Cheng, F.; Chen, J. Porous perovskite CaMnO3 as an electrocatalyst for rechargeable Li-O2 batteries. Chem. Commun. 2014, 50, 1497–1499.
- Jung, J.I.; Jeong, H.Y.; Lee, J.S.; Kim, M.G.; Cho, J. A bifunctional perovskite catalyst for oxygen reduction and evolution. Angew. Chem. 2014, 126, 4670–4674.
- Chen, C.-F.; King, G.; Dickerson, R.M.; Papin, P.A.; Gupta, S.; Kellogg, W.R.; Wu, G. Oxygen-deficient BaTiO3−x perovskite as an efficient bifunctional oxygen electrocatalyst. Nano Energy 2015, 13, 423–432.
- Cui, X.; Wu, T.; Gai, D.; Yang, C.; Ding, Y.; Zhao, P. Enhancement of perovskites performance for coal tar decomposition by pore structure and acid-base modification. Fuel 2023, 331, 125654.
- Lu, F.; Wang, Y.; Jin, C.; Li, F.; Yang, R.; Chen, F. Microporous La0.8Sr0.2MnO3 perovskite nanorods as efficient electrocatalysts for lithium-air battery. J. Power Sources 2015, 293, 726–733.
- Lee, Y.C.; Peng, P.Y.; Chang, W.S.; Huang, C.M. Hierarchical meso-macroporous LaMnO3 electrode material for rechargeable zinc–air batteries. J. Taiwan Inst. Chem. Eng. 2014, 45, 2334–2339.
- Wang, Y.; Cui, X.; Li, Y.; Chen, L.; Shu, Z.; Chen, H.; Shi, J. High surface area mesoporous LaFexCo1−xO3 oxides: Synthesis and electrocatalytic property for oxygen reduction. Dalton Trans. 2013, 42, 9448–9452.
- Yang, Y.; Zhou, W.; Liu, R.; Li, M.; Rufford, T.E.; Zhu, Z. In Situ tetraethoxysilane-templated porous Ba0. 5Sr0. 5Co0. 8Fe0. 2O3−δ perovskite for the oxygen evolution reaction. ChemElectroChem 2015, 2, 200–203.
- Sun, Y.; Zhang, Y.; Yang, Y.; Chen, J.; Hua, B.; Shi, Y.; Wang, C.; Luo, J. Smart tuning of 3D ordered electrocatalysts for enhanced oxygen reduction reaction. Appl. Catal. B Environ. 2017, 219, 640–644.
- Oh, M.Y.; Lee, J.J.; Zahoor, A.; Gnana kumar, G.; Nahm, K.S. Enhanced electrocatalytic activity of three-dimensionally-ordered macroporous La0.6Sr0.4CoO3−δ perovskite oxide for Li-O2 battery application. RSC Adv. 2016, 6, 32212–32219.
- Qiu, P.; Ma, B.; Hung, C.-T.; Li, W.; Zhao, D. Spherical mesoporous materials from single to multilevel architectures. Acc. Chem. Res. 2019, 52, 2928–2938.
- Su, X.; Sun, Y.; Jin, L.; Zhang, L.; Yang, Y.; Kerns, P.; Liu, B.; Li, S.; He, J. Hierarchically porous Cu/Zn bimetallic catalysts for highly selective CO2 electroreduction to liquid C2 products. Appl. Catal. B Environ. 2020, 269, 118800.
