Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Head and neck cancer (HNC) is among the ten most frequent tumours, with 5-year survival rates varying from 30% to 70% depending on the stage and location of the tumour. HNC is traditionally known as head and neck squamous cell carcinoma (HNSCC), since 90% arises from epithelial cells. Metastasis remains a major cause of mortality in patients with HNSCC. HNSCC patients with metastatic disease have an extremely poor prognosis with a survival rate of less than a year. Matrix metalloproteinases (MMPs) have been described as biomarkers that promote cell migration and invasion.
- head and neck cancer (HNC)
- matrix metalloproteinases (MMPs)
- prognosis
1. Introduction
Head and neck cancer (HNC) is among the ten most frequent types of tumours, occupying the sixth place. Worldwide, HNC accounts for more than 890,000 new cases and 450,000 deaths annually due to this cancer [1]. It is defined as a malignant neoplasm that is developed from the mucosal epithelium in the oral cavity, pharynx and larynx. There are many types of HNCs, which are categorized by their anatomical location following the International Classification of Diseases (ICD-10) from the World Health Organization (WHO). HNC is traditionally known as head and neck squamous cell carcinoma (HNSCC), since 90% arises from epithelial cells [2][3]. Its incidence has decreased significantly in countries within Asia, North America, Australia, and south and east of Europe. But it is increasing in several countries located in Eastern and Northern Europe among men, and in Southern and Western Europe among women, reflecting the tobacco epidemic. This fact contrasts with the decline in other Western countries where smoking cessation has been earlier. However, an increased incidence in oral cancer in sites related to HPV infection was found, which could be attributed to changes in sexual behaviour, tobacco, alcohol and diet [4][5]. According to EUROCARE, considering head and neck tumours as the most lethal tumours, are those located in the hypopharynx and larynx with 25% and 59%, respectively. This is followed by the oropharynx with 39%, tongue with 43%, oral cavity with 45% and nasopharynx with 49% [6].
Smoking is the most important risk factor for oral cancer. Tobacco consumption has become a global epidemic. One billion men smoke worldwide, with 35% in developed countries and 50% in developing countries. And over 250 million women are smokers, with 22% in developed countries and 9% in developing countries. This trend indicates that men are smoking less and women are increasing their consumption [7]. Alcohol consumption, the second most important risk factor, is associated with the risk of suffering from HNSCC [8]. It is widespread throughout the history of humanity and across the planet. All alcoholic products carry with them an increased risk of HNSCC [9].
Tumour location is one of the most important prognostic factors because it influences cancer’s ability to metastasize. Tumours of the floor of the mouth and those of the posterior 2/3 of the tongue have a greater capacity for migration through lymph nodes than those that originate in the gums and buccal mucosa. Those originating in the lips, both in the vermilion and dermal portions, have a low metastatic behaviour, compared to tongue cancer. However, the location is not the only determining factor and others are important, such as the stage of the tumour [10].
The treatment alternatives are surgery resecting the tumour, radiotherapy (external radiotherapy or brachytherapy or a combination of these treatments) and chemotherapy, depending on the stage at which the oral cancer is found and after evaluating the variables of the tumour size and its possibilities of propagation to distance. Early diagnosis is essential so that therapy is as simple and effective as possible, directly influencing the success of treatment [3].
Locoregional recurrence is a major cause of patient mortality and morbidity. Although radiation eradicates a large proportion of tumour cells, selected groups of tumour cells (clonogens) are able to survive and repopulate the irradiated areas. Tumours are known as radioresistant if recurrences are observed within six months after the first course of radiation. Tumours can become resistant to radiotherapy for several reasons. Cells proliferate very rapidly and recolonise between treatments and/or tumour cells exhibit mechanisms to become resistant to radiation, such as hypoxia (as oxygen is needed to increase the damage of radiotherapy to the cell’s DNA). In these cases, systemic treatment can be added, improving the results of radiotherapy [11]. Radioresistance is also associated with a high degree of molecular heterogeneity. Other possible molecular mechanisms of radioresistance are enhanced DNA damage repair capacity, increased reactive oxygen species (ROS) scavenging capacity, epithelial–mesenchymal transition (EMT) and abnormal regulation of programmed cell death, which have revealed new targets for the therapy of different types of tumours, including oral squamous cell carcinoma (OSCC) [12]. The role of cancer stem cells (CSCs) in radioresistance has also been described [12][13].
2. MMPs and HNC
2.1. Invasion and Metastasis
The role of MMPs in invasion and metastasis has been evaluated by various authors [14][15]. For invasion to occur, a critical step must take place, which is the signalling of the initiation of the metastatic cascade through the interaction of the cells of the tumour with BM. Tumour cells initiate ECM degradation behaviour through BM proteolysis, leading to tumour cell propagation [3][15]. The degradation of the ECM also leads to an important change in the phenotypic change of the cells, acquiring a mesenchymal profile due to a phenomenon called the epithelial–mesenchymal transition, controlled mainly by MMPs [16].
Görögh et al. [15] concluded that MMP-2 overexpression was correlated with metastasis and that high levels of TIMP-1and -2 decrease tumour growth. Ren et al. [17] found that the mRNAs of MMP-7, MMP-13 and MMP-10 were regulated and that MMP-12 and MMP-9 were not regulated in metastatic tumours compared to non-metastatic ones. This suggests that there are genes that play important roles in metastasis through the regulation of MMP-7 and MMP-13. Nishio et al. [18] found that there are higher levels of MMP-2 and MMP-9 at metastatic sites compared to those of the primary tumour. Also, there were statistical differences found in both T1 and T2 cases. As compared to the higher expression of MMPs in metastatic regions, tumour-associated macrophages (TAMs) were in the primary regions. Thus, it can be concluded that the number of TAM and MMP expression levels are expected to have an inverse relationship between the primary and metastatic regions. Chakraborty et al. [19] observed that in 56.2% cases, the expression of MMP-9 was more correlated with the presence of lymph node metastases, an advanced stage of cancer and grade of tumour. De Vicente et al. [20], in an observational study, also concluded that MMP-2 and MMP-9 are overexpressed in patients with lymph node involvement and that MMP-9 is related to a low survival rate and therefore a poor prognosis. Targeting the remodelling of the HNSCC microenvironment has been suggested as a therapeutic approach to preventing lymph node metastasis. In this sense, it has been found that MMP-9 and MMP-14 were upregulated in the metastatic lymph nodes and closely positively correlated with the level of ALG-2 interacting protein X (ALIX), a protein that promotes the migration and invasion of HNSCC cells. The expression of ALIX in HNSCC was analysed and demonstrated to be statistically higher than in normal mucosae. MMP-9 and MMP-14 were found in the cytoplasm of cancer cells and were significantly enhanced in metastatic lymph nodes compared to the primary tumour. The degradation of extracellular matrix caused by MMPs is critical and the results suggest that ALIX could contribute to increased MMP-9 and MMP-14, leading to lymph node metastasis. Data from The Cancer Genome Atlas (TCGA) further verified the correlation between ALIX and MMP-14, although there was no correlation with MMP-9 [21].
2.2. Epithelial–Mesenchymal Transition (EMT)
MMPs are a family of endopeptidases responsible for dissolving the ECM and the BM. Figure 1 summarises the main processes in which MMPs are involved. They are produced by various inflammatory and connective tissue cells such as the following: fibroblasts, lymphocytes, endothelial cells and macrophages. And its synthesis is strictly regulated by hormones, growth factors, cytokines and TIMPs [22].
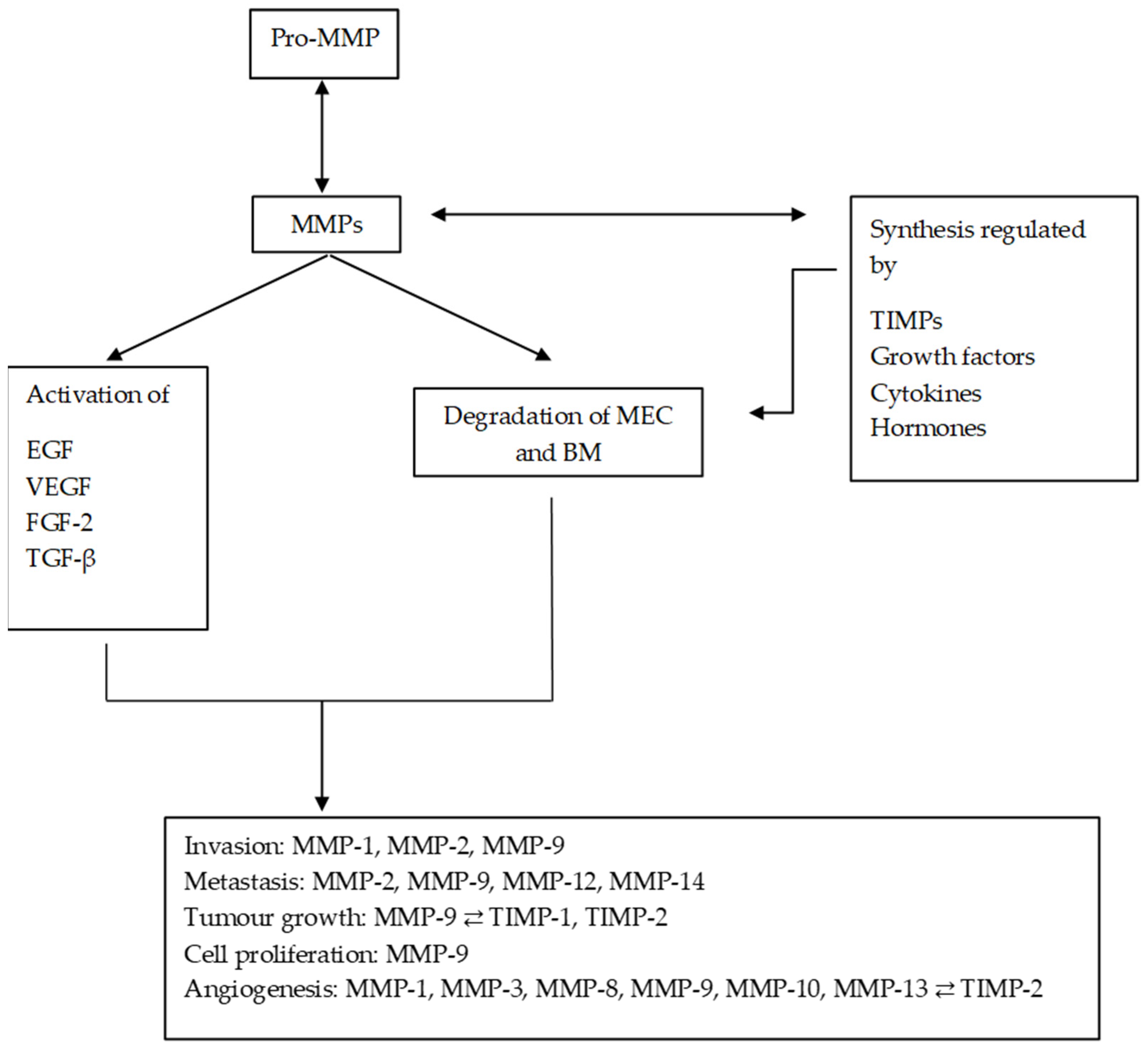
Figure 1. Diagram of the main processes in which MMPs are involved and their regulation by TIMPs. MMPs regulate the synthesis and activation of other substances, promoting and altering the tumour microenvironment. MMPs: matrix metalloproteinases; ECM: extracellular matrix; EGF: epithelial growth factor; VEGF: vascular endothelial growth factor; TGF-β: transforming growth factor beta; FGF-2: fibroblast growth factor 2.
Due to the role played by MMPs in the degradation process of BM and ECM, they have been considered as promising prognostic biomarkers in OSCC. Several studies have shown that the levels of MMPs are increased in cancer patients or patients with oral dysplasia compared to the control group. Smriti et al. [23] obtained a significant increase in salivary MMP-9 in patients with OSCC and oral dysplasia (OD) compared to the control group, and subjects with OD had higher levels than OSCC patients, suggesting greater expression of MMP-9 in direct relation to tumour development. Ghallab et al. [24] established that there was a direct relationship between the levels of MMP-9 and the malignancy of the lesions, for which they designed an observational and cross-sectional study that included 15 controls, 15 OSCC patients and 15 OD patients. MMP-1, MMP-2 and MMP-9 are overexpressed in OD and have the capacity to invade by breaking the BM and the ECM. Also, MMP-1 and MMP-9 were established as biomarkers that promote malignancy by Jordan et al. They studied the expression of MMP-1, MMP-2 and MMP-9 through an experimental study using TaqMan reverse transcription polymerase chain reaction (RT-PCR) in 34 oral dysplasia patients and 15 OSCC patients. The majority of dysplasias became OSCC. The MMP-1 and -9 levels were significantly higher in the OSCC cases compared with the dysplasias (p = 0.004 and p = 0.01). At the same time, MMP-1 and -9 mRNA levels were also significantly higher in the oral dysplasias that turned into oral cancer compared with those that did not [25].
2.3. Tumour Growth
An active migration of tumour cells from the original tissue must happen before metastasis and this is carried out by degrading the BM. This membrane constitutes the first barrier for invasion to occur; one of its major components is type IV collagen. MMP-2 is secreted as a zymogen, but in contact with membrane-type matrix metalloproteinase-1 (MT1-MMP) or MMP-14, it is activated [26]. And it begins to degrade BM, which allows for the expansion of the cancer, which subsequently requires an induction of angiogenesis in tumour tissue to nourish proliferating tumour cells [27]. Several studies have associated an overexpression of MMP-9 with the progression of oral cancer and a poor prognosis [27]. Liu et al. concluded that mRNA expression levels of MMPs were significantly higher in HNSCC than in normal tissues, declaring that they could serve as a therapeutic target and prognostic biomarker in HNSCC [28][29].
De Vicente et al. [22] showed how MMP-9 is related to TNM parameters and its expression was associated with a poor prognosis. Zheng et al., through a meta-analysis that involved nine case–control studies that combined 419 patients with oral cancer, demonstrated that there was poorer overall survival in patients who tested positive for MMP-9 compared to those who were negative for MMP-9 in the non-activity-based subgroup. Significant differences in MMP-9 expression were observed between patients with OSCC and metastasis and those without metastasis (p < 0.05). Indeed, a significantly higher expression of MMP-9 has been shown in tumours with invasive T3 and T4 stages compared to T1 and T2 tumours [30].
2.4. Angiogenesis
Angiogenesis is a critical process in tumour formation and progression. Different signalling cascades have been revealed to be involved in tumour neovascularization, such as VEGF. Hypoxia is a common factor in solid tumours, contributing to cancer progression and poor outcome. A large number of pro-angiogenic factors are regulated by hypoxia, including VEGF, platelet growth factors derived from factor β, the inhibitor of plasminogen activation, etc. [31].
The angiogenic process begins with the recruitment of blood vessels in response to the release of growth factors and cytokines by tumour cells and the secretion of proteases such as MMP-9 [32]. In fact, it is known that the blood vessels of a tumour mass are formed due to various processes, including the appearance of endothelial cells from neighbouring capillaries and small blood vessels in response to the release of growth factors and the recruitment of progenitor cells from the tumour mass of the bone marrow. In addition, tumour cells can also form vascular channels and express endothelial biomarkers. This is a process called vascular mimicry, described by Maniotis et al., and all of these tumour vascularization mechanisms require the activity of MMPs, mainly MMP-1, MMP-2, MMP-9 and MMP-14 [33].
In addition to their pro-angiogenic function, MMPs may also function in anti-angiogenic activity. In fact, a variety of endogenous angiogenic inhibitors are derived from the MMP-mediated degradation of ECM molecules. These inhibitors are angiostatin, a fragment of plasminogen; endostatin, a fragment of type XVIII collagen; and tumstatin, derived from collagen IVα [32]. The accumulation of tumour-associated macrophages (TAMs) in the cancer stroma is correlated with angiogenesis in oral cancer [18]. TAMs also increase the secretion of angiogenic factors such as vascular endothelial growth factor and contribute to the integrity of the vascular structure of the tumour. Nishio et al. found a higher expression of MMP-2 and MMP-9 in the metastatic regions in OSSC patients. These results also showed a greater number of macrophages associated with the primary tumour, which would be responsible for promoting angiogenesis. These results may indicate that the tumour cells in the metastatic region still maintain invasive potential, whereas the TAMs in the primary region may play other roles such as in angiogenesis.
Normal vasculature consists of the interaction of two cell types, endothelial cells and surrounding pericytes, which share a common basement membrane and communicate by physical contact and paracrine signalling. These interactions are necessary for the survival, maturation and stabilization of the vascular system [34]. In contrast, tumour-associated blood vessels show heterogeneous function and anatomy and often become deficient and highly permeable, allowing macromolecules to escape [35]. This occurs in response to the factor responsible for vascular permeability produced by tumour cells. This increased permeability of tumour blood vessels is recognized as a first step in pathological and physiological angiogenesis, which is very similar in healing and inflammation processes [36]. The permeability produced by the structural basis of tumour-associated blood vessels is associated with defects in endothelial cells that form gaps between cells. In addition, defective endothelial cells composed of branches are disorganised and with the loss of intercellular connections, the pericytes that cover the vessels are also abnormal. These abnormalities include the loss of association with the endothelium and an abnormal morphology, associated with deep cytoplasmic projections that enter the tumour [37].
Tumour progression and metastasis depend on the formation and recruitment of new blood vessels in response to the release of proangiogenic factors, such as fibroblast growth factor 2, VEGF and IL-8. However, the overexpression of angiogenic factors such as HGF and placental growth factor (PIGF) has also been shown in the tissue and saliva of patients with OSCC. Moreover, MMP-1, MMP-3, MMP-8, MMP-9, MMP-10, MMP-13 and TIMP-2 were significantly upregulated in the saliva of OSCC patients compared to healthy controls. The concept of a dominance of anti-angiogenic factors, including inhibitors of angiogenesis such as thrombospondin and TIMPs, is replaced by the abundance of pro-angiogenic factors before the initiation of tumour neovascularisation and is known as the angiogenic switch [38].
This entry is adapted from the peer-reviewed paper 10.3390/ijms25010527
References
- International Agency for Research on Cancer (IACR). International Association of Cancer Registries. Available online: http://ci5.iarc.fr/CI5plus/Default.aspx (accessed on 31 March 2020).
- Moore, S.R.; Pierce, A.M.; Wilson, D.F. ‘Oral cancer’—The terminology dilemma. Oral Dis. 2000, 6, 191–193.
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894.
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108.
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48.
- Gatta, G.; Botta, L.; Sánchez, M.J.; Anderson, L.A.; Pierannunzio, D.; Licitra, L.; EUROCARE Working Group. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer 2015, 51, 2130–2143.
- Petti, S. Lifestyle risk factors for oral cancer. Oral Oncol. 2009, 45, 340–350.
- Auperin, A.; Melkane, A.; Luce, D.; Temam, S. Épidémiologie des cancers des voies aérodigestives supérieures. Lett. Cancerol. 2011, 10, 102–106.
- Baron, A.E.; Francheschi, S.; Barra, S.; Talamini, R.; La Vecchia, C. A comparison of the Joint Effects of Alcohol and Smoking on the Risk of Cancer across Sites in the Upper Aerodigestive Tract. Cancer Epidemiol. Biomark. Prev. 1993, 2, 519–523.
- Gallegos-Hernández, J.F.; Abrego-Vázquez, J.A. Factores pronóstico en cáncer de boca. Acta Méd. Grupo Angeles 2010, 8, 88–94.
- Glenny, A.M.; Furness, S.; Worthington, H.V.; Conway, D.I.; Oliver, R.; Clarkson, J.E.; Macluskey, M.; Pavitt, S.; Kelvin Kw Chan, K.K.; Brocklehurst, P.; et al. Interventions for the treatment of oral cavity and oropharyngeal cancer: Radiotherapy. Cochrane Database Syst. Rev. 2010, 12, CD006387.
- Feller, G.; Khammissa, R.A.G.; Nemutandani, M.S.; Feller, L. Biological consequences of cancer radiotherapy in the context of oral squamous cell carcinoma. Head Face Med. 2021, 17, 35.
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Marchal, J.A.; Núñez, M.I. CSC radioresistance: A therapeutic challenge to improve radiotherapy effectiveness in cancer. Cells 2020, 9, 1651.
- Singh, R.D.; Haridas, N.; Patel, J.B.; Shah, F.D.; Shukla, S.N.; Shah, P.M.; Patel, P.S. Metalloproteinases and their inhibitors: Correlation with invasion and metastasis in oral cancer. Indian J. Clin. Biochem. 2010, 25, 250–259.
- Görögh, T.; Beier, U.H.; Bäumken, J.; Meyer, J.E.; Hoffmann, M.; Gottschlich, S.; Maune, S. Metalloproteinases and their inhibitors: Influence on tumour invasiveness and metastasis formation in head and neck squamous cell carcinomas. Head Neck 2006, 28, 31–39.
- Mughees, M.; Sengupta, A.; Khowal, S.; Wajid, S. Mechanism of tumour microenvironment in the progression and development of oral cancer. Mol. Biol. Rep. 2021, 48, 1773–1786.
- Ren, Z.H.; Wu, K.; Yang, R.; Liu, Z.Q.; Cao, W. Differential expression of matrix metalloproteinases and miRNAs in the metastasis of oral squamous cell carcinoma. BMC Oral Health 2020, 20, 24.
- Nishio, K.; Motozawa, K.; Omagari, D.; Takahiro Gojoubori, T.; Ikeda, T.; Asano, M.; Gionhaku, N. Comparison of MMP2 and MMP9 expression levels between primary and metastatic regions of oral squamous cell carcinoma. J. Oral Sci. 2016, 58, 59–65.
- Chakraborty, S.; Suresh, T.; Mohiyuddin, A.S. Role of Matrix metalloproteinase 9 in predicting lymph node metastases in oral squamous cell carcinoma. Cureus 2023, 15, e33495.
- de Vicente, J.C.; Lequerica-Fernández, P.; López-Arranz, J.S.; Esteban, I.; Fresno, M.F.; Astudillo, A. Expression of matrix metalloproteinase-9 in high-grade salivary gland carcinomas is associated with their metastatic potential. Laryngoscope 2008, 118, 247–251.
- Xie, Q.H.; Wang, W.M.; Yang, J.G.; Xia, H.F.; Xiao, B.L.; Chen, G.H.; Huang, J.; Li, R.F.; Chen, G. ALIX promotes cell migration and invasion of head and neck squamous cell carcinoma by regulating the expression of MMP9, MMP14, VEGF-C. Arch. Oral Biol. 2023, 151, 105696.
- Ricci, S.; Pinto, F.; Auletta, A.; Giordano, A.; Giovane, A.; Settembre, G.; Boccellino, M.; Boffo, S.; Di Carlo, A.; Di Domenico, M. The enigmatic role of matrix metalloproteinases in epithelial-to-mesenchymal transition of oral squamous cell carcinoma: Implications and nutraceutical aspects. J. Cell. Biochem. 2019, 120, 6813–6819.
- Smriti, K.; Ray, M.; Chatterjee, T.; Shenoy, R.P.; Gadicherla, S.; Pentapati, K.C.; Rustaqi, N. Salivary MMP-9 as a Biomarker for the Diagnosis of Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2020, 21, 233–238.
- Ghallab, N.A.; Shaker, O.G. Serum and salivary levels of chemerin and MMP-9 in oral squamous cell carcinoma and oral premalignant lesions. Clin. Oral Investig. 2017, 21, 937–947.
- Jordan, R.C.K.; Macabeo-Ong, M.; Shiboski, C.H.; Dekker, N.; Ginzinger, D.G.; Wong, D.T.W.; Brian, L.; Schmidt, B.I. Overexpression of Matrix Metalloproteinase-1 and -9 mRNA Is Associated with Progression of Oral Dysplasia to Cancer. Clin. Cancer Res. 2004, 10, 6460–6465.
- Sato, H.; Takino, T.; Okada, Y.; Cao, J.; Shinagawa, A.; Yamamoto, E.; Seiki, M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994, 370, 61–65.
- Aznavoorian, S.; Murphy, A.N.; Stetler-Stevenson, W.G.; Liotta, L.A. Molecular aspects of tumour cell invasion and metastasis. Cancer 1993, 71, 1368–1383.
- Piao, S.; Zhao, S.; Guo, F.; Xue, J.; Yao, G.; Wei, Z.; Huang, Q.; Sun, Y.; Zhang, B. Increased expression of CD147 and MMP-9 is correlated with poor prognosis of salivary duct carcinoma. J. Cancer Res. Clin. Oncol. 2012, 138, 627–635.
- Liu, M.; Huang, L.; Liu, Y.; Yang, S.; Rao, Y.; Chen, X.; Nie, M.; Liu, X. Identification of the MMP family as therapeutic targets and prognostic biomarkers in the microenvironment of head and neck squamous cell carcinoma. J. Transl. Med. 2023, 21, 208.
- Zheng, W.Y.; Zhang, D.T.; Yang, S.Y.; Li, H. Elevated matrix metalloproteinase-9 expression correlates with advanced stages of oral cancer and is linked to poor clinical outcomes. J. Oral Maxillofac. Surg. 2015, 73, 2334–2342.
- Ma, T.T.; Wang, L.; Wang, J.L.; Liu, Y.J.; Chen, Y.C.; He, H.J.; Song, Y. Hypoxia-induced cleavage of soluble ephrinA1 from cancer cells is mediated by MMP-2 and associates with angiogenesis in oral squamous cell carcinoma. OncoTargets Ther. 2019, 12, 8491–8499.
- Shuman Moss, L.A.; Jensen-Taubman, S.; Stetler-Stevenson, W.G. Matrix Metalloproteinases. Am. J. Pathol. 2012, 181, 1895–1899.
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752.
- Bodnar, R.J.; Rodgers, M.E.; Chen, W.C.W.; Wells, A. Pericyte regulation of vascular remodeling through the CXC receptor 3. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2818–2829.
- Dvorak, H.F.; Nagy, J.A.; Dvorak, J.T.; Dvorak, A.M. Identification and characterization of the blood vessels of solid tumours that are leaky to circulating macromolecules. Am. J. Pathol. 1988, 133, 95–109.
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039.
- Morikawa, S.; Baluk, P.; Kaidoh, T.; Haskell, A.; Jain, R.K.; McDonald, D.M. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumours. Am. J. Pathol. 2002, 160, 985–1000.
- Cai, M.; Zheng, Z.; Bai, Z.; Ouyang, K.; Wu, Q.; Xu, S.; Huang, L.; Jiang, Y.; Wang, L.; Gao, J.; et al. Overexpression of angiogenic factors and matrix metalloproteinases in the saliva of oral squamous cell carcinoma patients: Potential non-invasive diagnostic and therapeutic biomarkers. BMC Cancer 2022, 22, 530.
This entry is offline, you can click here to edit this entry!
