Subarachnoid hemorrhage (SAH) resulting from the rupture of an intracranial aneurysm (IA) is a devastating type of stroke affecting around 6/100,000 patients worldwide annually [
1], leading to high mortality and morbidity rates [
2,
3]. It harbors a case fatality rate of 50% [
4]. Due to the young age of onset compared to ischemic stroke and intracerebral hemorrhage, SAH is a major contributor to the stroke-related loss of productive life years despite advancements in risk assessment, imaging techniques, and surgical and intensive care treatment [
2,
3]. Most SAH survivors suffer from persistent, disabling neurological deficits; even those who experience some degree of neurological recovery often face ongoing psychological and cognitive impairments. As a result, 46% of SAH survivors remain severely disabled in their activities of daily life and are unable to return to work, resulting in a considerable socioeconomic burden [
3,
5]. Approximately 3% of the population harbors an incidental IA, but only a minority will experience a rupture leading to aneurysmal SAH [
6]. While various risk factors for IA rupture have been identified, including smoking, prior SAH, hypertension, hypercholesterolemia, age, gender, aneurysm location, aneurysm size, heart disease, and aspirin use [
7,
8,
9,
10,
11,
12], their respective individual impact is far from being fully investigated [
13,
14,
15].
2. Exploring Pathways: Inflammation and Cerebral Aneurysm Formation, Gut–Brain Interactions, and Microbiome Analysis
2.1. The Role of Inflammation in Cerebral Aneurysm Formation
Increasing evidence suggests that inflammation plays a pivotal role in the formation of IAs [
33]. This process includes endothelial dysfunction, followed by an inflammatory response, the phenotype shift of smooth muscle cells (SMCs), the remodeling of the extracellular matrix, and ultimately, cell death and degradation of the vessel wall [
34,
35]. The initial cause of endothelial dysfunction and subsequent vascular remodeling is the result of wall shear stress [
36]. It was shown that areas of high wall shear stress, such as the apex of an arterial bifurcation, are especially predisposed to aneurysm formation [
37]. Mechanical shear stress upregulates the expression of pro-inflammatory mediators, such as the nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) [
38], matrix metalloproteinases (MMPs) [
39], interleukin-1β (IL-1β) [
40], Ets-1, and monocyte chemoattractant protein-1 (MCP-1) [
41] and downregulates the expression of anti-inflammatory mediators, such as nitric oxide (NO) [
40] in endothelial cells. Pro-inflammatory mediators activate the inflammatory response, in which macrophages play a pivotal role [
33]. Macrophages not only release pro-inflammatory cytokines that attract more inflammatory cells, but also secrete MMPs that break down the extracellular matrix of the arterial wall, causing additional damage by promoting the activation of other proteinases. For rats, it was shown that the presence of macrophages and their derived MMPs was closely linked to IA growth, and that the inhibition of these MMPs haltered the progression of IAs [
42]. Similarly, Kanematsu et al. [
32] found that mice depleted of macrophages had a significantly reduced risk of developing IAs. Moreover, inhibiting MCP-1, a chemokine that controls the infiltration of macrophages, prevented the development of IAs in mice [
43].
Macrophages are not the sole cells participating in the inflammatory response within the IA wall.
SMCs, primarily located in the media layer of vessels, are the primary cells responsible for producing the extracellular matrix in the vascular wall [
33]. During the early stages of aneurysm formation, SMCs migrate from the media layer into the intima layer in response to endothelial injury and undergo proliferation, resulting in myointimal hyperplasia. As the process continues, SMCs undergo a phenotypic shift from a specialized phenotype focused on contraction to a dedifferentiated phenotype, which contributes to inflammation and the breakdown of the extracellular matrix by expressing pro-inflammatory mediators and MMPs [
34]. Morphologically, these dedifferentiated SMCs no longer maintain their tightly compacted spindle-like arrangement, but instead separate from each other and take on a spider-like appearance within the aneurysm walls, leading to remodeling [
46].
MMPs are observed to be produced by both macrophages [
42] and SMCs [
45] within the wall of the blood vessels or aneurysms. These MMPs play a role in breaking down the extracellular matrix of the arterial wall, leading to additional damage through the upregulation of other proteinases and angiogenic factors [
47].
Given this crucial role of inflammation in the pathophysiology of IA formation, the gut and oral microbiome could also be involved in this process by modulating the inflammatory response.
2.2. Potential Mechanisms of Gut–Brain Interaction
In rats, after ischemic stroke, an intestinal dysregulation with a greater permeability of the gut-blood barrier has been shown [
48]. Consecutively, lipopolysaccharide (LPS) from Gram-negative bacteria of the intestine is translocated to the systemic circulation [
49], activating inflammatory processes. Following cerebral ischemia, the disruption of the blood-brain barrier permits the entry of LPS into the brain parenchyma. This, in turn, triggers the activation of Toll-like receptor 4 (TLR4) and the release of inflammatory cytokines, further intensifying the damage to the ischemic brain [
50]. Not only after ischemic stroke, but also after intracerebral hemorrhage (ICH), intestinal permeability increased in mice [
51]. Moreover, T cells and monocytes originating from intestinal Peyer’s patches accumulated in the intracerebral hematoma. The expression of pro-inflammatory markers like IL-1β, inducible nitric oxide synthase, and tumor necrosis factor α (TNF-α) was significantly elevated in the brain tissue, while this was reversed after fecal microbiota transplantation.
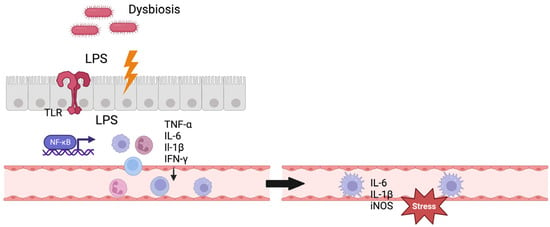
For IA formation, the gut–brain interaction still remains unclear. A direct translocation of bacteria or LPS to the IAs appears unlikely [
52]; instead, an indirect mechanism modulating the inflammatory response in the aneurysm wall is proposed [
53] (
Figure 1). Other potential mechanisms of gut–brain interaction include the direct stimulation of the enteric and autonomic nervous system, neuroendocrine pathways, and the production of biochemical (neuro-)transmitters by microbiota [
49].
Figure 1. Gut–brain interaction. Figure shows a potential mechanism of gut–brain interaction. Gut dysbiosis leads to dysregulation of the gut–blood barrier and LPS translocation to systemic circulation, consecutively activating the immune system. The immune cells enter the intracranial vessels through the systemic circulation and exert stress on the vascular endothelium here through inflammatory mediators. TLR = Toll-like receptor, IL = interleukin, TNF = tumor necrosis factor, iNOS = inducible nitric oxide synthase, IFN = interferon. Created with BioRender.com.
2.3. Methods of Analyzing the Microbiome
The two currently predominant approaches for microbial identification in microbiome samples involve the next-generation sequencing (NGS) of gene amplicons from marker genes, such as 16S rRNA, or shotgun metagenomics [
54].
16S-rRNA gene amplicon sequencing: This approach is a targeted approach, i.e., with the help of the polymerase chain reaction (PCR), a marker gene of interest is amplified. The amplicons are then sequenced in high throughput and the sequences are used to identify an organism. The primary target for bacterial identification is normally the 16S-rRNA gene [
54]. Due to its critical role in the ribosome, it is a well-conserved gene and suitable for the taxonomic classification of bacteria [
55]. The 16S-rRNA gene sequence can be divided into invariable regions and nine variable regions (V1–V9). PCR is used with specific primers, which bind in the conserved regions.
Metagenome sequencing: This approach is an untargeted approach; i.e., where possible, the complete DNA of a given sample is isolated, fragmented, and sequenced (shotgun sequencing; [
54]). Due to its untargeted nature, it could, in principle, detect all organisms present; however, this is limited by sequencing depth. For instance, biopsies might contain too-low numbers of bacteria and their DNA is “drowned” in human DNA. In contrast, in stool samples, where primarily only bacteria are found, one can uncover the genes, pathways, and metabolic functions existing within the community [
62]. However, this still is limited, since the function of about 40–60% of the genes present in a given sample cannot be functionally predicted [
63]. Nevertheless, deep-sequenced metagenome samples are certainly helpful in detecting bacteria, which might cause or have caused an aneurysm (see below).
3. The Gut Microbiome and Intracranial Aneurysm Formation and Rupture
While several studies have investigated the microbiome’s influence on stroke [
17,
18,
19,
64], there are limited data on the role of the microbiome in IA formation and rupture. The studies discussed here are depicted in
Table 1.
Table 1. Overview of studies on the gut microbiome and IAs.
|
Study
|
Type
|
Medium
|
Intervention
|
Aim
|
Method
|
Result
|
|
Shikata et al., 2019 [52]
|
interventional study
|
mice
|
gut depletion by antibiotics in mice with IA induction vs. mice with normal gut and IA induction
|
- number and rupture rate of IAs;
- number of macrophages in IA tissue;
- mRNA levels of cytokines in IA tissue.
|
- immunohistochemistry;
- RT-PCR.
|
- gut depletion reduced the incidence of IA (83% vs. 6%, p < 0.001) and rupture;
- macrophage infiltration and mRNA levels of inflammatory cytokines were reduced with gut depletion.
|
|
Li et al., 2020 [65]
|
case–control study
|
- humans
- mice
|
- analysis of fecal samples of 140 UIA and 140 control patients;
- 20 mice treated with UIA patient feces and 20 treated
with control feces.
|
- comparison of gut microbiome of patients with UIAs and without;
- test, if changes in the gut microbiota influence the progression of UIAs in vivo.
|
- metagenomic shotgun sequencing;
- serum metabolomic analysis.
|
- Bacteroides ssp., Odoribacter splanchnicus, Clostridium ssp. were significantly enriched in the UIAs;
- Hungatella hathewayi was enriched in the control group;
- microbiome of UIAs was significantly dominated by unsaturated fatty acid biosynthesis;
- microbiome of controls was dominated by amino acid synthesis;
- treatment with feces from UIA patients increased the overall incidence of IAs (85% vs. 45%; p = 0.019) and rupture rate (82% vs. 22%; p = 0.009);
- serum concentrations of 2 of 8 fatty acids and 8 of 38 amino acids differed in mice transplanted with feces from UIA patients and controls.
|
|
Kawabata et al., 2022. [66]
|
multicenter, prospective case–control
|
humans
|
analysis of fecal samples of 28 RAs vs. 33 UIAs
|
comparison of gut microbiome of patients with UIAs and RAs
|
16S rRNA sequencing
|
- gut microbiome profile of UIAs and RAs were significantly different;
- Campylobacter ssp. and Campylobacter ureolyticus were significantly higher in the RA group.
|
|
He et al., 2023.
[67]
|
two-sample Mendelian
randomization study
|
humans
|
database analysis of gut microbiome of patients with IA, UIA, SAH
|
association between the gut microbiome and the risk of
IA, UIA, and SAH
|
inverse variance weighting approach
|
- Candidatus Soleaferrea decreased the risk of IA;
- Holdemania and Olsenella increased risk of IA;
- Lentisphaeria, Porphyromonadaceae, Bilophila, Fusicatenibacter, Ruminococcus sp. 1,
Victivallales decreased risk of SAH;
- Streptococcaceae increased risk of SAH;
- Porphyromonadaceae, Bilophila decreased the risk of UIA;
- Oxalobacteraceae, Adlercreutzia,
Intestinimonas, Victivallis increased the risk of UIA.
|
|
Ma et al., 2023.
[68]
|
two-sample Mendelian
randomization study
|
humans
|
database analysis of gut microbiome of UIA patients
|
association between the gut microbiome and the risk of
UIA
|
inverse variance weighting approach
|
- Clostridia, Rhodospirillaceae, Adlercreutzia, Sutterella, Victivallis, Streptococcus, Peptostreptococcaceae increased risk of UIA;
- Oscillospira, Paraprevotella decreased the risk of UIA.
|
IA = intracranial aneurysm, UIA = unruptured intracranial aneurysm, RA = ruptured intracranial aneurysm, SAH = subarachnoid hemorrhage, RT-PCR = real time polymerase chain reaction, sp. = species (sg.); ssp. = species (pl.).
4. The Oral Microbiome and Intracranial Aneurysm Formation and Rupture
The literature on the role of the oral microbiome in intracranial aneurysm formation is limited. Table 2 gives an overview.
Table 2. Overview of studies on the oral microbiome and IAs.
|
Study
|
Type
|
Medium
|
Intervention
|
Aim
|
Method
|
Result
|
|
Pyysalo et al., 2013. [71]
|
prospective cohort study
|
humans
|
analysis of RA tissue of 36 patients with SAH
|
assess the presence of oral and pharyngeal bacterial
genome in RAs
|
qRT-PCR
|
- bacterial DNA was detected in 21/36 (58%);
- DNA from endodontic bacteria was detected in 20/36 (56%) and from periodontal bacteria in 17/36 (47%);
- DNA of the Streptococcus-mitis group was the most common.
|
|
Pyysalo et al., 2016. [72]
|
prospective cohort study
|
humans
|
analysis of RA tissue of 42 patients and UIA tissue of 28 patients, tissue from healthy vessels and cardiac by-pass operations as controls
|
assess the presence of oral and pharyngeal bacterial DNA in RAs and UIAs
|
qRT-PCR
|
- bacterial DNA was detected in 49/70 (70%);
- 29/42 (69%) of the RA tissue and 20/28 (71%) of the UIA tissue contained bacterial DNA of oral origin;
- RA and UIA samples contained significantly more bacterial DNA than control samples.
|
|
Pyysalo et al., 2018. [73]
|
prospective cohort study
|
humans
|
analysis of tissue from gingival pockets of 30 patients with RA and 60 with UIA
|
assess the presence of dental infectious foci and odontogenic bacteria in patients
before surgical treatment of IA
|
qRT-PCR
|
- total of 43% had gingival pockets of 6 mm or deeper;
- bacterial and Fusobacterium nucleatum DNA were significantly higher in the patients with ≥6 mm gingival pockets than patients without them.
|
|
Inenaga et al., 2018. [74]
|
prospective cohort study
|
humans
|
analysis of saliva from 48 patients with CES, 151 with non-CES infarct, 54 with ICH, 43 with RA, and 97 with UIA vs. 79 healthy controls
|
assess the rate of Streptococcus mutans
with collagen-binding protein, Cnm, in CES, non-CES infarct, ICH, RA, and UIA
|
PCR
|
- significantly high Cnm-positive rate was observed in CES, non-CES infarct, ICH and RA compared to controls.
|
|
Aboukais et al., 2019. [75]
|
prospective cohort study
|
humans
|
analysis of IA tissue from 10 patients with RA and 20 with UIA, samples from STA, dura mater, and MCA as control
|
assess the presence of bacteria in the walls of UIAs and RAs
|
PCR
|
- no bacterial presence was found in the wall of aneurysms.
|
|
Hallikainen et al., 2019. [76]
|
case series, case–control, prospective study
|
humans
|
oral examination of
42 patients with UIAs and 34 RAs compared to 5170 from prospective database
|
association of periodontitis with IA formation and SAH
|
multivariate logistic regression
|
- periodontitis, severe periodontitis, and gingival bleeding increased the risk of IAs significantly;
severe periodontitis in ≥3 teeth or gingival bleeding increased the risk of SAH significantly.
|
|
Hallikainen et al., 2021. [77]
|
prospective cohort study
|
humans
|
analysis of serum of 227 IA patients, compared to 1096 from prospective database
|
association of IgA and IgG against Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans with IA and SAH
|
ELISA
|
- high IgA against P. gingivalis and A. actinomycetemcomitans increased the risk of IA and SAH significantly;
- high IgG levels against P. gingivalis and A. actinomycetemcomitans decreased the risk of IA and SAH significantly.
|
|
Hallikainen et al., 2023. [78]
|
case–control, prospective study
|
humans
|
oral examination of 60 patients with UIA and 30 with RA compared to 5144 from prospective database
|
association of caries with IA formation and SAH
|
multivariate logistic regression
|
- caries does not increase the risk of IAs and SAH.
|
RA = ruptured intracranial aneurysm, UIA = unruptured intracranial aneurysm, SAH = subarachnoid hemorrhage, ICH = intracerebral hemorrhage, CES = cardioembolic stroke, qRT-PCR = real time quantitative polymerase chain reaction, STA = superficial temporal artery, MCA = middle meningeal artery, sp. = species (sg.), ssp. = species (pl.).