One of the main concerns related to SARS-CoV-2 infection is the symptoms that could be developed by survivors, known as long COVID, a syndrome characterized by persistent symptoms beyond the acute phase of the infection. This syndrome has emerged as a complex and debilitating condition with a diverse range of manifestations affecting multiple organ systems. It is increasingly recognized for affecting the Central Nervous System, in which one of the most prevalent manifestations is cognitive impairment. The search for effective therapeutic interventions has led to growing interest in Mesenchymal Stem Cell (MSC)-based therapies due to their immunomodulatory, anti-inflammatory, and tissue regenerative properties.
- long COVID
- mesenchymal stem cells
- exosomes
- neurological sequelae
1. Introduction
2. SARS-CoV-2 Neuroinvasiveness and Long COVID
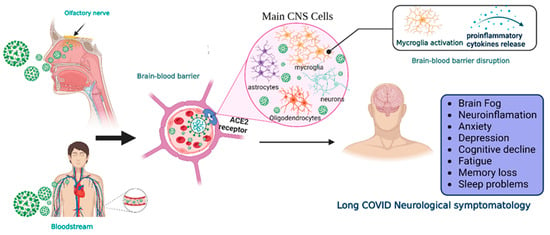
3. Current Landscape of MSC and MSC-Derived Exosomes in Long COVID
4. Administration Routes of MSC and MSC-Derived Exosomes for Neurological Diseases
This entry is adapted from the peer-reviewed paper 10.3390/biom14010008
References
- Li, Y.; Bai, W.; Hashikawa, T. The Neuroinvasive Potential of SARS-CoV2 May Play a Role in the Respiratory Failure of COVID-19 Patients. J. Med. Virol. 2020, 92, 552–555.
- Santos-López, G.; Cortés-Hernández, P.; Vallejo-Ruiz, V.; Reyes-Leyva, J. SARS-CoV-2: Basic Concepts, Origin and Treatment Advances. Gac. Med. Mex. 2021, 157, 84–89.
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 14 November 2023).
- Loke, X.Y.; Imran, S.A.M.; Tye, G.J.; Wan Kamarul Zaman, W.S.; Nordin, F. Immunomodulation and Regenerative Capacity of MSCs for Long-COVID. Int. J. Mol. Sci. 2021, 22, 12421.
- Priyal; Sehgal, V.; Kapila, S.; Taneja, R.; Mehmi, P.; Gulati, N. Review of Neurological Manifestations of SARS-CoV-2. Cureus 2023, 15, e38194.
- Román-Montes, C.M.; Flores-Soto, Y.; Guaracha-Basañez, G.A.; Tamez-Torres, K.M.; Sifuentes-Osornio, J.; González-Lara, M.F.; de León, A.P. Post-COVID-19 Syndrome and Quality of Life Impairment in Severe COVID-19 Mexican Patients. Front. Public Health 2023, 11, 1155951.
- World Health Organization a Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 14 November 2023).
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An Overview. Diabetes Metab. Syndr. 2021, 15, 869–875.
- Natarajan, A.; Shetty, A.; Delanerolle, G.; Zeng, Y.; Zhang, Y.; Raymont, V.; Rathod, S.; Halabi, S.; Elliot, K.; Shi, J.Q.; et al. A Systematic Review and Meta-Analysis of Long COVID Symptoms. Syst. Rev. 2023, 12, 88.
- Zhao, Y.; Lukiw, W.J. SARS-CoV-2 Neuroinvasion, Inflammatory Neurodegeneration and Alzheimer’s Disease. Front. Cell. Neurosci. 2022, 16, 937961.
- Chapoval, S.P.; Keegan, A.D. Perspectives and Potential Approaches for Targeting Neuropilin 1 in SARS-CoV-2 Infection. Mol. Med. 2021, 27, 162.
- Masre, S.F.; Jufri, N.F.; Ibrahim, F.W.; Abdul Raub, S.H. Classical and Alternative Receptors for SARS-CoV-2 Therapeutic Strategy. Rev. Med. Virol. 2021, 31, 1–9.
- Xia, H.; Lazartigues, E. Angiotensin-Converting Enzyme 2: Central Regulator for Cardiovascular Function. Curr. Hypertens. Rep. 2010, 12, 170–175.
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C.A.; Choi, A.; Cho, S.-M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12, 816.
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175.
- Bauer, L.; Laksono, B.M.; de Vrij, F.M.S.; Kushner, S.A.; Harschnitz, O.; van Riel, D. The Neuroinvasiveness, Neurotropism, and Neurovirulence of SARS-CoV-2. Trends Neurosci. 2022, 45, 358–368.
- Norouzi, M.; Miar, P.; Norouzi, S.; Nikpour, P. Nervous System Involvement in COVID-19: A Review of the Current Knowledge. Mol. Neurobiol. 2021, 58, 3561–3574.
- Reza-Zaldívar, E.E.; Hernández-Sapiéns, M.A.; Minjarez, B.; Gómez-Pinedo, U.; Márquez-Aguirre, A.L.; Mateos-Díaz, J.C.; Matias-Guiu, J.; Canales-Aguirre, A.A. Infection Mechanism of SARS-COV-2 and Its Implication on the Nervous System. Front. Immunol. 2020, 11, 621735.
- Erickson, M.A.; Rhea, E.M.; Knopp, R.C.; Banks, W.A. Interactions of SARS-CoV-2 with the Blood–Brain Barrier. Int. J. Mol. Sci. 2021, 22, 2681.
- Stefanou, M.-I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C.; et al. Neurological Manifestations of Long-COVID Syndrome: A Narrative Review. Ther. Adv. Chronic Dis. 2022, 13, 204062232210768.
- Monje, M.; Iwasaki, A. The Neurobiology of Long COVID. Neuron 2022, 110, 3484–3496.
- Whittaker, A.; Anson, M.; Harky, A. Neurological Manifestations of COVID-19: A Systematic Review and Current Update. Acta Neurol. Scand. 2020, 142, 14–22.
- Acharya, A.; Kevadiya, B.D.; Gendelman, H.E.; Byrareddy, S.N. SARS-CoV-2 Infection Leads to Neurological Dysfunction. J. Neuroimmune Pharmacol. 2020, 15, 167–173.
- Desforges, M.; Le Coupanec, A.; Brison, E.; Meessen-Pinard, M.; Talbot, P.J. Neuroinvasive and Neurotropic Human Respiratory Coronaviruses: Potential Neurovirulent Agents in Humans. Adv. Exp. Med. Biol. 2014, 807, 75–96.
- Asadi-Pooya, A.A.; Simani, L. Central Nervous System Manifestations of COVID-19: A Systematic Review. J. Neurol. Sci. 2020, 413, 116832.
- Doyle, M.F. Central Nervous System Outcomes of COVID-19. Transl. Res. 2022, 241, 41–51.
- Chen, X.; Laurent, S.; Onur, O.A.; Kleineberg, N.N.; Fink, G.R.; Schweitzer, F.; Warnke, C. A Systematic Review of Neurological Symptoms and Complications of COVID-19. J. Neurol. 2021, 268, 392–402.
- van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.L.B.J.; van Jaarsveld, C.H.M. Post-Acute and Long-COVID-19 Symptoms in Patients with Mild Diseases: A Systematic Review. Fam. Pract. 2022, 39, 159–167.
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-Term Neurologic Outcomes of COVID-19. Nat. Med. 2022, 28, 2406–2415.
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-Month Neurological and Psychiatric Outcomes in 236 379 Survivors of COVID-19: A Retrospective Cohort Study Using Electronic Health Records. Lancet Psychiatry 2021, 8, 416–427.
- Yong, S.J.; Liu, S. Proposed Subtypes of Post-COVID-19 Syndrome (or Long-COVID) and Their Respective Potential Therapies. Rev. Med. Virol. 2022, 32, e2315.
- Delgado-Alonso, C.; Valles-Salgado, M.; Delgado-Álvarez, A.; Yus, M.; Gómez-Ruiz, N.; Jorquera, M.; Polidura, C.; Gil, M.J.; Marcos, A.; Matías-Guiu, J.; et al. Cognitive Dysfunction Associated with COVID-19: A Comprehensive Neuropsychological Study. J. Psychiatr. Res. 2022, 150, 40–46.
- Hugon, J.; Msika, E.-F.; Queneau, M.; Farid, K.; Paquet, C. Long COVID: Cognitive Complaints (Brain Fog) and Dysfunction of the Cingulate Cortex. J. Neurol. 2022, 269, 44–46.
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long Covid-Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648.
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-Acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169.
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708.
- Tutal Gursoy, G.; Yuksel, H.; Mulkem Simsek, I.; Oral, S.; Erdogan Kucukdagli, F.; Karaman, A.; Akinci, E.; Bastug, A.; Guner, H.R.; Bektas, H. Neurological Presentations in Patients with COVID-19 in Cytokine Storm. Can. J. Neurol. Sci./J. Can. des Sci. Neurol. 2023, 50, 89–95.
- Jarrott, B.; Head, R.; Pringle, K.G.; Lumbers, E.R.; Martin, J.H. “LONG COVID”—A Hypothesis for Understanding the Biological Basis and Pharmacological Treatment Strategy. Pharmacol. Res. Perspect. 2022, 10, e00911.
- Thepmankorn, P.; Bach, J.; Lasfar, A.; Zhao, X.; Souayah, S.; Chong, Z.Z.; Souayah, N. Cytokine Storm Induced by SARS-CoV-2 Infection: The Spectrum of Its Neurological Manifestations. Cytokine 2021, 138, 155404.
- Díez-Cirarda, M.; Yus, M.; Gómez-Ruiz, N.; Polidura, C.; Gil-Martínez, L.; Delgado-Alonso, C.; Jorquera, M.; Gómez-Pinedo, U.; Matias-Guiu, J.; Arrazola, J.; et al. Multimodal Neuroimaging in Post-COVID Syndrome and Correlation with Cognition. Brain 2023, 146, 2142–2152.
- Díez-Cirarda, M.; Yus-Fuertes, M.; Sanchez-Sanchez, R.; Gonzalez-Rosa, J.J.; Gonzalez-Escamilla, G.; Gil-Martínez, L.; Delgado-Alonso, C.; Gil-Moreno, M.J.; Valles-Salgado, M.; Cano-Cano, F.; et al. Hippocampal Subfield Abnormalities and Biomarkers of Pathologic Brain Changes: From SARS-CoV-2 Acute Infection to Post-COVID Syndrome. EBioMedicine 2023, 94, 104711.
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 Is Associated with Changes in Brain Structure in UK Biobank. Nature 2022, 604, 697–707.
- Blomberg, B.; Cox, R.J.; Langeland, N. Long COVID: A Growing Problem in Need of Intervention. Cell Rep. Med. 2022, 3, 100552.
- Delgado-Alonso, C.; Díez-Cirarda, M.; Pagán, J.; Pérez-Izquierdo, C.; Oliver-Mas, S.; Fernández-Romero, L.; Martínez-Petit, Á.; Valles-Salgado, M.; Gil-Moreno, M.J.; Yus, M.; et al. Unraveling Brain Fog in Post-COVID Syndrome: Relationship between Subjective Cognitive Complaints and Cognitive Function, Fatigue, and Neuropsychiatric Symptoms. Eur. J. Neurol. 2023; Online ahead of print.
- Chrzanowski, W.; Kim, S.Y.; McClements, L. Can Stem Cells Beat COVID-19: Advancing Stem Cells and Extracellular Vesicles Toward Mainstream Medicine for Lung Injuries Associated with SARS-CoV-2 Infections. Front. Bioeng. Biotechnol. 2020, 8, 554.
- Zayed, M.; Iohara, K. Immunomodulation and Regeneration Properties of Dental Pulp Stem Cells: A Potential Therapy to Treat Coronavirus Disease 2019. Cell Transplant. 2020, 29, 096368972095208.
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451.
- Iyer, S.S.; Rojas, M. Anti-Inflammatory Effects of Mesenchymal Stem Cells: Novel Concept for Future Therapies. Expert Opin. Biol. Ther. 2008, 8, 569–581.
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286.
- Silva, J.D.; Lopes-Pacheco, M.; Paz, A.H.R.; Cruz, F.F.; Melo, E.B.; de Oliveira, M.V.; Xisto, D.G.; Capelozzi, V.L.; Morales, M.M.; Pelosi, P.; et al. Mesenchymal Stem Cells From Bone Marrow, Adipose Tissue, and Lung Tissue Differentially Mitigate Lung and Distal Organ Damage in Experimental Acute Respiratory Distress Syndrome*. Crit. Care Med. 2018, 46, e132–e140.
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Influenza Virus-Induced Acute Lung Injury in a Pig Model. Stem Cell Res. Ther. 2018, 9, 17.
- Loy, H.; Kuok, D.I.T.; Hui, K.P.Y.; Choi, M.H.L.; Yuen, W.; Nicholls, J.M.; Peiris, J.S.M.; Chan, M.C.W. Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus—Associated Acute Lung Injury. J. Infect. Dis. 2019, 219, 186–196.
- Khoury, M.; Cuenca, J.; Cruz, F.F.; Figueroa, F.E.; Rocco, P.R.M.; Weiss, D.J. Current Status of Cell-Based Therapies for Respiratory Virus Infections: Applicability to COVID-19. Eur. Respir. J. 2020, 55, 2000858.
- Rogers, C.J.; Harman, R.J.; Bunnell, B.A.; Schreiber, M.A.; Xiang, C.; Wang, F.-S.; Santidrian, A.F.; Minev, B.R. Rationale for the Clinical Use of Adipose-Derived Mesenchymal Stem Cells for COVID-19 Patients. J. Transl. Med. 2020, 18, 203.
- Liang, B.; Chen, J.; Li, T.; Wu, H.; Yang, W.; Li, Y.; Li, J.; Yu, C.; Nie, F.; Ma, Z.; et al. Clinical Remission of a Critically Ill COVID-19 Patient Treated by Human Umbilical Cord Mesenchymal Stem Cells. Medicine 2020, 99, e21429.
- Shu, L.; Niu, C.; Li, R.; Huang, T.; Wang, Y.; Huang, M.; Ji, N.; Zheng, Y.; Chen, X.; Shi, L.; et al. Treatment of Severe COVID-19 with Human Umbilical Cord Mesenchymal Stem Cells. Stem Cell Res. Ther. 2020, 11, 361.
- Fathi-Kazerooni, M.; Fattah-Ghazi, S.; Darzi, M.; Makarem, J.; Nasiri, R.; Salahshour, F.; Dehghan-Manshadi, S.A.; Kazemnejad, S. Safety and Efficacy Study of Allogeneic Human Menstrual Blood Stromal Cells Secretome to Treat Severe COVID-19 Patients: Clinical Trial Phase I & II. Stem Cell Res. Ther. 2022, 13, 96.
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754.
- Zhu, Y.-G.; Shi, M.-M.; Monsel, A.; Dai, C.-X.; Dong, X.; Shen, H.; Li, S.-K.; Chang, J.; Xu, C.-L.; Li, P.; et al. Nebulized Exosomes Derived from Allogenic Adipose Tissue Mesenchymal Stromal Cells in Patients with Severe COVID-19: A Pilot Study. Stem Cell Res. Ther. 2022, 13, 220.
- Zarrabi, M.; Shahrbaf, M.A.; Nouri, M.; Shekari, F.; Hosseini, S.-E.; Hashemian, S.-M.R.; Aliannejad, R.; Jamaati, H.; Khavandgar, N.; Alemi, H.; et al. Allogenic Mesenchymal Stromal Cells and Their Extracellular Vesicles in COVID-19 Induced ARDS: A Randomized Controlled Trial. Stem Cell Res. Ther. 2023, 14, 169.
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity after Stroke in Rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715.
- Monsel, A.; Hauw-Berlemont, C.; Mebarki, M.; Heming, N.; Mayaux, J.; Nguekap Tchoumba, O.; Diehl, J.-L.; Demoule, A.; Annane, D.; Marois, C.; et al. Treatment of COVID-19-Associated ARDS with Mesenchymal Stromal Cells: A Multicenter Randomized Double-Blind Trial. Crit. Care 2022, 26, 48.
- Bowdish, M.E.; Barkauskas, C.E.; Overbey, J.R.; Gottlieb, R.L.; Osman, K.; Duggal, A.; Marks, M.E.; Hupf, J.; Fernandes, E.; Leshnower, B.G.; et al. A Randomized Trial of Mesenchymal Stromal Cells for Moderate to Severe Acute Respiratory Distress Syndrome from COVID-19. Am. J. Respir. Crit. Care Med. 2023, 207, 261–270.
- Kaffash Farkhad, N.; Sedaghat, A.; Reihani, H.; Adhami Moghadam, A.; Bagheri Moghadam, A.; Khadem Ghaebi, N.; Khodadoust, M.A.; Ganjali, R.; Tafreshian, A.R.; Tavakol-Afshari, J. Mesenchymal Stromal Cell Therapy for COVID-19-Induced ARDS Patients: A Successful Phase 1, Control-Placebo Group, Clinical Trial. Stem Cell Res. Ther. 2022, 13, 283.
- Karyana, M.; Djaharuddin, I.; Rif’ati, L.; Arif, M.; Choi, M.K.; Angginy, N.; Yoon, A.; Han, J.; Josh, F.; Arlinda, D.; et al. Safety of DW-MSC Infusion in Patients with Low Clinical Risk COVID-19 Infection: A Randomized, Double-Blind, Placebo-Controlled Trial. Stem Cell Res. Ther. 2022, 13, 134.
- Wang, F.; Tang, H.; Zhu, J.; Zhang, J.H. Transplanting Mesenchymal Stem Cells for Treatment of Ischemic Stroke. Cell Transplant. 2018, 27, 1825–1834.
- Cui, Y.; Ma, S.; Zhang, C.; Cao, W.; Liu, M.; Li, D.; Lv, P.; Xing, Q.; Qu, R.; Yao, N.; et al. Human Umbilical Cord Mesenchymal Stem Cells Transplantation Improves Cognitive Function in Alzheimer’s Disease Mice by Decreasing Oxidative Stress and Promoting Hippocampal Neurogenesis. Behav. Brain Res. 2017, 320, 291–301.
- Bao, X.; Wei, J.; Feng, M.; Lu, S.; Li, G.; Dou, W.; Ma, W.; Ma, S.; An, Y.; Qin, C.; et al. Transplantation of Human Bone Marrow-Derived Mesenchymal Stem Cells Promotes Behavioral Recovery and Endogenous Neurogenesis after Cerebral Ischemia in Rats. Brain Res. 2011, 1367, 103–113.
- Oh, S.H.; Kim, H.N.; Park, H.-J.; Shin, J.Y.; Lee, P.H. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Neuronal Differentiation by Enhancing the Wnt Signaling Pathway in an Alzheimer’s Disease Model. Cell Transplant. 2015, 24, 1097–1109.
- Martins, L.F.; Costa, R.O.; Pedro, J.R.; Aguiar, P.; Serra, S.C.; Teixeira, F.G.; Sousa, N.; Salgado, A.J.; Almeida, R.D. Mesenchymal Stem Cells Secretome-Induced Axonal Outgrowth Is Mediated by BDNF. Sci. Rep. 2017, 7, 4153.
- Lin, L.; Lin, H.; Bai, S.; Zheng, L.; Zhang, X. Bone Marrow Mesenchymal Stem Cells (BMSCs) Improved Functional Recovery of Spinal Cord Injury Partly by Promoting Axonal Regeneration. Neurochem. Int. 2018, 115, 80–84.
- Shahror, R.A.; Linares, G.R.; Wang, Y.; Hsueh, S.-C.; Wu, C.-C.; Chuang, D.-M.; Chiang, Y.-H.; Chen, K.-Y. Transplantation of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 Facilitates Cognitive Recovery and Enhances Neurogenesis in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2020, 37, 14–26.
- Mitsialis, S.A.; Kourembanas, S. Stem Cell–Based Therapies for the Newborn Lung and Brain: Possibilities and Challenges. Semin. Perinatol. 2016, 40, 138–151.
- Harrell, C.R.; Volarevic, A.; Djonov, V.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes as New Remedy for the Treatment of Neurocognitive Disorders. Int. J. Mol. Sci. 2021, 22, 1433.
- Teixeira, F.; Salgado, A. Mesenchymal Stem Cells Secretome: Current Trends and Future Challenges. Neural Regen. Res. 2020, 15, 75.
- Reza-Zaldivar, E.; Hernández-Sapiéns, M.; Gutiérrez-Mercado, Y.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.; Vázquez-Méndez, E.; Padilla-Camberos, E.; Canales-Aguirre, A. Mesenchymal Stem Cell-Derived Exosomes Promote Neurogenesis and Cognitive Function Recovery in a Mouse Model of Alzheimer’s Disease. Neural Regen. Res. 2019, 14, 1626.
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Minjarez, B.; Gutiérrez-Mercado, Y.K.; Márquez-Aguirre, A.L.; Canales-Aguirre, A.A. Potential Effects of MSC-Derived Exosomes in Neuroplasticity in Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 317.
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536.
- Vizoso, F.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852.
- Luarte, A.; Bátiz, L.F.; Wyneken, U.; Lafourcade, C. Potential Therapies by Stem Cell-Derived Exosomes in CNS Diseases: Focusing on the Neurogenic Niche. Stem Cells Int. 2016, 2016, 5736059.
- Cantinieaux, D.; Quertainmont, R.; Blacher, S.; Rossi, L.; Wanet, T.; Noël, A.; Brook, G.; Schoenen, J.; Franzen, R. Conditioned Medium from Bone Marrow-Derived Mesenchymal Stem Cells Improves Recovery after Spinal Cord Injury in Rats: An Original Strategy to Avoid Cell Transplantation. PLoS ONE 2013, 8, e69515.
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Paracrine-Mediated Neuroprotection and Neuritogenesis of Axotomised Retinal Ganglion Cells by Human Dental Pulp Stem Cells: Comparison with Human Bone Marrow and Adipose-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e109305.
- Kupcova Skalnikova, H. Proteomic Techniques for Characterisation of Mesenchymal Stem Cell Secretome. Biochimie 2013, 95, 2196–2211.
- Skalnikova, H.; Motlik, J.; Gadher, S.J.; Kovarova, H. Mapping of the Secretome of Primary Isolates of Mammalian Cells, Stem Cells and Derived Cell Lines. Proteomics 2011, 11, 691–708.
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692.
- Ma, X.; Huang, M.; Zheng, M.; Dai, C.; Song, Q.; Zhang, Q.; Li, Q.; Gu, X.; Chen, H.; Jiang, G.; et al. ADSCs-Derived Extracellular Vesicles Alleviate Neuronal Damage, Promote Neurogenesis and Rescue Memory Loss in Mice with Alzheimer’s Disease. J. Control. Release 2020, 327, 688–702.
- Yang, Y.; Ye, Y.; Su, X.; He, J.; Bai, W.; He, X. MSCs-Derived Exosomes and Neuroinflammation, Neurogenesis and Therapy of Traumatic Brain Injury. Front. Cell. Neurosci. 2017, 11, 55.
- Ding, M.; Shen, Y.; Wang, P.; Xie, Z.; Xu, S.; Zhu, Z.Y.; Wang, Y.; Lyu, Y.; Wang, D.; Xu, L.; et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 2165–2177.
- Shi, J.; Zhao, Y.-C.; Niu, Z.-F.; Fan, H.-J.; Hou, S.-K.; Guo, X.-Q.; Sang, L.; Lv, Q. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles in the Treatment of Human Diseases: Progress and Prospect. World J. Stem Cells 2021, 13, 49–63.
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from Mesenchymal Stem/Stromal Cells: A New Therapeutic Paradigm. Biomark. Res. 2019, 7, 8.
- Grumet, M.; Sherman, J.; Dorf, B.S. Efficacy of MSC in Patients with Severe COVID-19: Analysis of the Literature and a Case Study. Stem Cells Transl. Med. 2022, 11, 1103–1112.
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in Mesenchymal Stem Cell Clinical Trials 2004–2018: Is Efficacy Optimal in a Narrow Dose Range? Stem Cells Transl. Med. 2020, 9, 17–27.
- Masterson, C.H.; Curley, G.F.; Laffey, J.G. Modulating the Distribution and Fate of Exogenously Delivered MSCs to Enhance Therapeutic Potential: Knowns and Unknowns. Intensiv. Care Med. Exp. 2019, 7, 41.
- Kim, S.Y.; Chrzanowski, W. Stem Cell Delivery Systems and Devices—Spraying. In Stem Cell-Based Therapy for Lung Disease; Springer International Publishing: Cham, Switzerland, 2019; pp. 241–253. ISBN 978-3-030-29403-8.
- Jamshidi, E.; Babajani, A.; Soltani, P.; Niknejad, H. Proposed Mechanisms of Targeting COVID-19 by Delivering Mesenchymal Stem Cells and Their Exosomes to Damaged Organs. Stem Cell Rev. Rep. 2021, 17, 176–192.
- Lee, N.K.; Yang, J.; Chang, E.H.; Park, S.E.; Lee, J.; Choi, S.J.; Oh, W.; Chang, J.W.; Na, D.L. Intra-Arterially Delivered Mesenchymal Stem Cells Are Not Detected in the Brain Parenchyma in an Alzheimer’s Disease Mouse Model. PLoS ONE 2016, 11, e0155912.
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int. 2013, 2013, 732742.
- Van den Bos, J.; Ouaamari, Y.E.; Wouters, K.; Cools, N.; Wens, I. Are Cell-Based Therapies Safe and Effective in the Treatment of Neurodegenerative Diseases? A Systematic Review with Meta-Analysis. Biomolecules 2022, 12, 340.
- Deng, L.; Peng, Q.; Wang, H.; Pan, J.; Zhou, Y.; Pan, K.; Li, J.; Wu, Y.; Wang, Y. Intrathecal Injection of Allogenic Bone Marrow-Derived Mesenchymal Stromal Cells in Treatment of Patients with Severe Ischemic Stroke: Study Protocol for a Randomized Controlled Observer-Blinded Trial. Transl. Stroke Res. 2019, 10, 170–177.
- Kim, H.; Na, D.L.; Lee, N.K.; Kim, A.R.; Lee, S.; Jang, H. Intrathecal Injection in a Rat Model: A Potential Route to Deliver Human Wharton’s Jelly-Derived Mesenchymal Stem Cells into the Brain. Int. J. Mol. Sci. 2020, 21, 1272.
- Barmada, A.; Sharan, J.; Band, N.; Prodromos, C. Serious Adverse Events Have Not Been Reported with Spinal Intrathecal Injection of Mesenchymal Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2023, 18, 829–833.
- Patel, M.M.; Patel, B.M. Crossing the Blood–Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133.
- Inoue, T.; Sugiyama, M.; Hattori, H.; Wakita, H.; Wakabayashi, T.; Ueda, M. Stem Cells from Human Exfoliated Deciduous Tooth-Derived Conditioned Medium Enhance Recovery of Focal Cerebral Ischemia in Rats. Tissue Eng. Part A 2013, 19, 24–29.
- Lochhead, J.J.; Thorne, R.G. Intranasal Delivery of Biologics to the Central Nervous System. Adv. Drug Deliv. Rev. 2012, 64, 614–628.
- Galeano, C.; Qiu, Z.; Mishra, A.; Farnsworth, S.L.; Hemmi, J.J.; Moreira, A.; Edenhoffer, P.; Hornsby, P.J. The Route by Which Intranasally Delivered Stem Cells Enter the Central Nervous System. Cell Transplant. 2018, 27, 501–514.
- Baak, L.M.; Wagenaar, N.; van der Aa, N.E.; Groenendaal, F.; Dudink, J.; Tataranno, M.L.; Mahamuud, U.; Verhage, C.H.; Eijsermans, R.M.J.C.; Smit, L.S.; et al. Feasibility and Safety of Intranasally Administered Mesenchymal Stromal Cells after Perinatal Arterial Ischaemic Stroke in the Netherlands (PASSIoN): A First-in-Human, Open-Label Intervention Study. Lancet Neurol. 2022, 21, 528–536.
