Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Excess body weight constitutes one of the major health challenges for societies and healthcare systems worldwide. Besides the type of diet, calorie intake and the lack of physical exercise, data have highlighted a possible association between endocrine-disrupting chemicals (EDCs), such as bisphenol A, phthalates and their analogs, and obesity. EDCs represent a heterogeneous group of chemicals that may influence the hormonal regulation of body mass and adipose tissue morphology.
- bisphenol
- body mass index
- endocrine disruptors
- endocrine-disrupting chemicals
- obesity
- phthalate
1. Introduction
Over recent decades, the escalating global increase in overweight and obesity, also known as “globesity”, has constituted one of the major health challenges for societies and healthcare systems worldwide. Based on the World Obesity Atlas 2023 report, approximately 38% of the world population presents with excess body weight, having a body mass index (BMI) of more than 25 kg/m2 [1]. This global prevalence of overweight and obesity is expected to reach 51% by 2035, while 78% of US adults are estimated to live with excess body weight by 2030 [2][3]. More striking than the elevated obesity rates in adults is the higher prevalence of excess body weight among children and adolescents, which has doubled or tripled in children of school age in many developed regions of the world [3][4]. Moreover, obesity is associated with a plethora of comorbidities, including diabetes mellitus type 2 (T2DM), coronary heart disease, hypertension, stroke, dyslipidemia, sleep apnea, osteoarthritis and some types of cancer [5][6][7][8].
During the past few decades, several research teams have investigated the association between common chemical exposures and the occurrence of allergy, asthma, immune dysfunction, cancer and other entities, including obesity [9][10]. The contemporary industrialized environment in developed countries is a constant source of a variety of chemical substances, presenting persistence and bioaccumulation potency in the food chain. Moreover, unrecognized or little-recognized environmental chemicals, such as industrial endocrine-disrupting chemicals (EDCs) or endocrine disruptors, have the potential to disrupt the actions of hormones, causing adverse health effects. Particularly, some EDCs that have been termed “obesogens” may also influence adipogenesis and regulatory metabolic pathways, leading to an imbalance in the regulation of body weight resulting in weight gain and obesity [11]. The most important EDCs that have been suspected in the development of obesity and obesity-associated metabolic disorders are the plastic additive bisphenol A (BPA) and the phthalate-based plasticizers.
Recent data from observational, animal and experimental studies have shown that certain chemical substances, such as EDCs, may have an impact on the endocrine system, being involved in the development and rapid propagation of obesity [12]. Based on the definition by the “Global assessment on the state of the science of endocrine disruptors” of the World Health Organization (WHO) in 2002, an endocrine disruptor has been described as “an exogenous substance or mixture that alters functions of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations” [13]. Certain EDCs are found in nature, such as phytoestrogens; however, the majority of EDCs are synthetic compounds that have been released through human activities into the ecosystem. Humans are exposed to endocrine disruptors through various sources in their daily lives, in outdoor and indoor environments and via the use of personal care and household products, industrial chemicals, pharmaceuticals, pesticides, herbicides, fungicides and flame retarders; air pollution; and dietary habits. EDCs constitute a highly heterogeneous group of chemical substances that can be categorized according to their chemical structure and properties, their occurrence and intended use, their mechanism of action, the potential direct or indirect impact on the endocrine system, the accumulation in the organism, their environmental persistence and the described or suggested clinical implications [14]. The catalog of EDCs encompasses agrochemicals, such as pesticides, herbicides and fungicides; industrial organic solvents/lubricants and their byproducts (dioxins, polychlorinated bisphenyls, polybrominated bisphenyls); pharmaceutical substances; catalysts; and plastic contaminants and plasticizers, such as bisphenols and phthalates [15]. In comparison to other EDCs, bisphenols and phthalates are metabolized and excreted relatively quickly (i.e., half-lives less than 24 h) and hence are considered nonpersistent [16]. Despite their nonpersistent nature, their ubiquitous and frequent use in a wide variety of consumer products throughout life leads to a chronic exposure, reported to be global [17].
Potential targets of EDCs include any endocrine organ, hormonal system and/or hormonally affected pathway [18]. Some of these EDCs may interfere with the regulation of metabolism, energy balance and the storage of fat in the organism, leading to the development of obesity by affecting the function of adipose tissue and disrupting metabolic endocrine signaling [19]. Figure 1 depicts some EDCs that have been implicated in obesity. Some of the best-documented groups of EDCs with obesogenic properties and widespread exposure in the general population are bisphenols and phthalates, which are mainly found in plastic products.
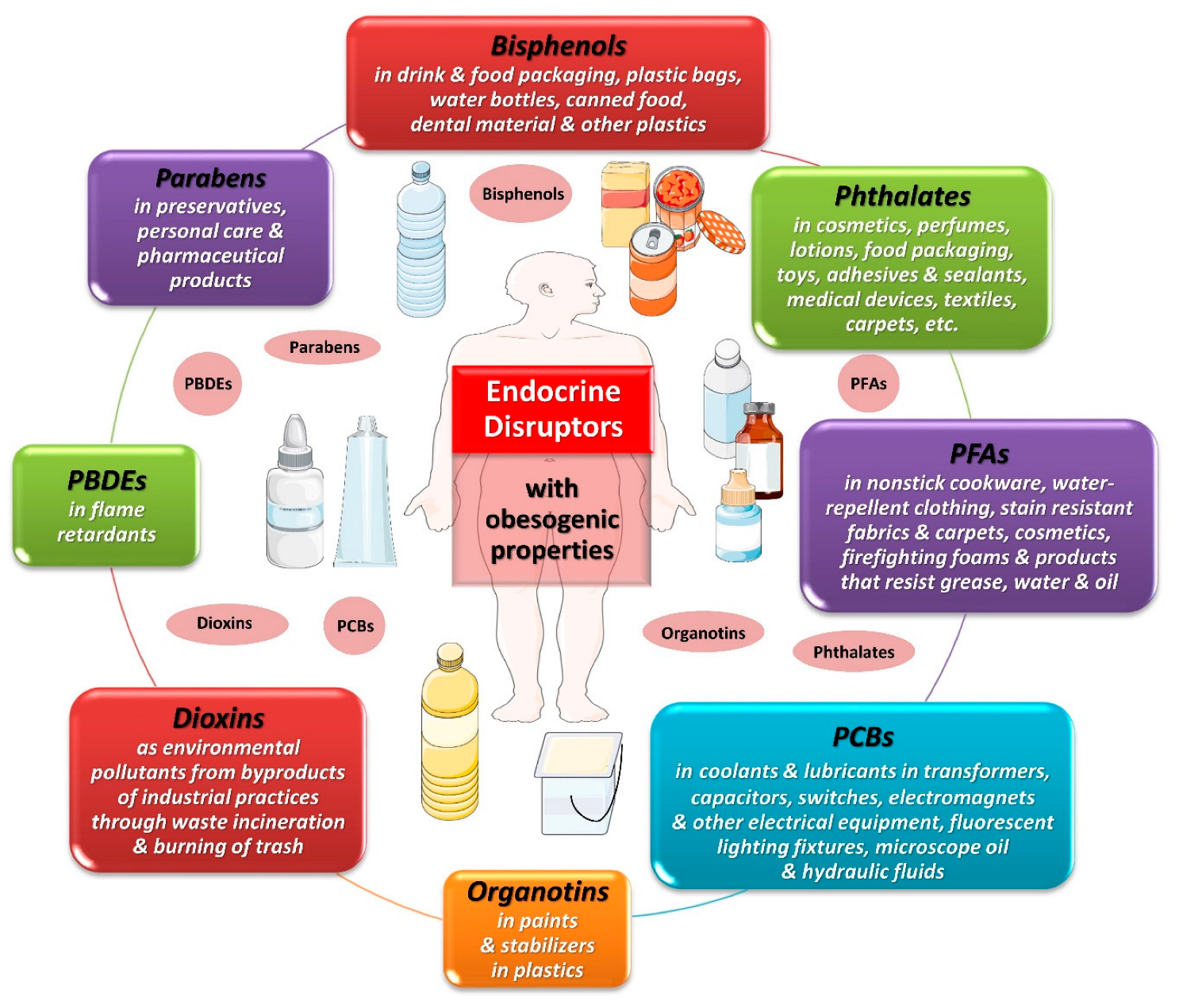
Figure 1. Obesogenic endocrine-disrupting chemicals. Abbreviations: PBDEs: polybrominated diphenyl ethers; PCBs: polychlorinated biphenyls; PFAs: perfluoroalkyl substances. All images are originated from the free medical site http://smart.servier.com/ (accessed on 1 December 2023) by Servier licensed under a Creative Commons Attribution 3.0 Unported License.
Globally, the production of plastics has increased significantly since the mid-20th century (Figure 2). After a stagnation in 2020 due to the emergence of the COVID-19 pandemic, this production reached 390.7 million tons in 2021 [20]. Interestingly, there was a parallel striking epidemic rise in obesity/overweight globally, as depicted in Figure 2, mainly attributed to the international food production and supply system [3][21]. Since 1970, food production and supply with ameliorated manufacturing and distribution systems have radically shifted in the direction of elevated energy availability [3].
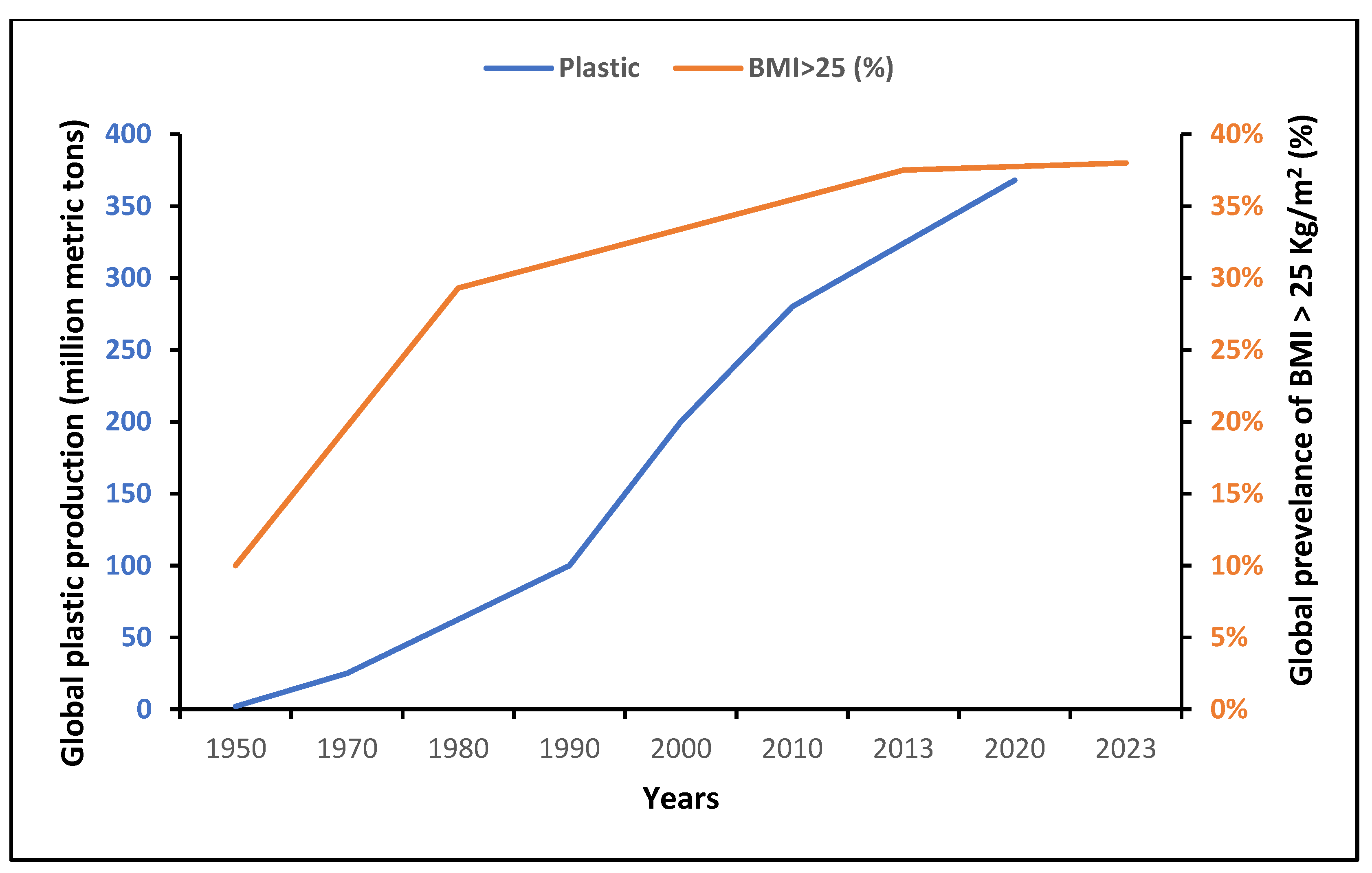
Worldwide, more people have access to palatable, cheap and ultra-processed foods of lower nutritional quality, and a number of obesogenic EDCs have gradually reached the global food chain, with a potential influence on human metabolism. Moreover, plasticizers are the main additives in the manufacture of plastic products, commonly used in the production of food packaging materials to improve flexibility, durability, processing and resistance to heat, fire and UV radiation. All these chemicals may be detached from polymer compounds and leak into the surrounding environment after being degraded. Humans may be exposed to those EDCs through various routes including food ingestion, the respiratory tract and dermal exposure [10].
2. Bisphenol A
Bisphenol A (BPA), or 4,4 -isopropylidenediphenol 2,2-bis (4-hydroxy-phenyl)- propane, was first reported by the chemist A.P. Dianin in 1891. It is a functional diphenyl compound that possesses two hydroxyl groups in the “para” position (Figure 3), which allows it to bind with androgen and estrogen receptors (classical nuclear ERα and ERβ, ERγ and membrane-associated GPR30) as an antagonist or agonist [22]. BPA is classified as a xenoestrogen due to its similarity with diethylstilbestrol, a synthetic estrogen, and as an EDC which enhances the ER with lower affinity compared to 17β-estradiol [23][24].
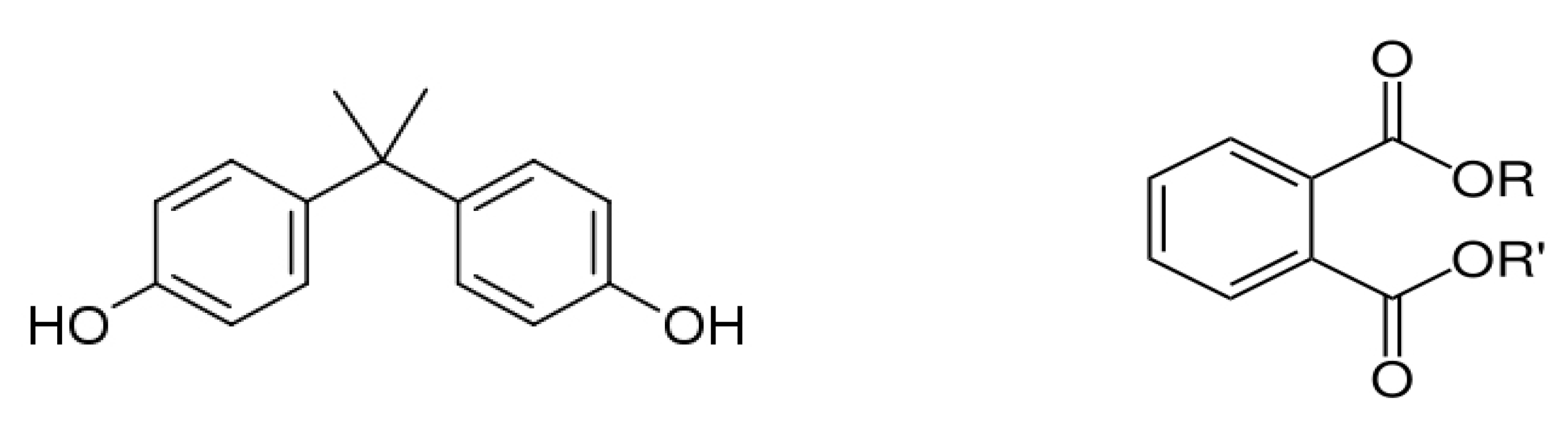
Figure 3. Chemical structure of bisphenol A (left) and phthalates (right).
Due to its elastic, cross-linking, polymer-forming and intrinsic heat resistance properties, BPA represents one of the world’s most heavily produced synthetic industrial chemical compounds listed by the Organization for Economic Cooperation and Development, with more than 15 billion pounds produced worldwide annually [25][26][27] and over 1 million pounds leached into the environment [28]. BPA is used worldwide in the synthesis of polycarbonate plastics, plastic consumer products, drink and food packaging, plastic bags, water bottles, epoxy resin linings of beverage containers and canned food, dental materials such as sealants, electronic equipment, toys, optical lenses, paper coatings, adhesives, dye developers and thermal papers [29].
BPA can be released into the surrounding environment through various means including exposure to heat or acidic conditions, hydrolysis or degradation of the polymer and constant diffusion of residual BPA that remains on the polymer [30]. The quantity of released BPA is affected by duration of exposure, processing methodology and environmental conditions such as temperature and PH [31]. Human exposure to nanomolar concentrations of environmental BPA is continuous and widespread via oral, skin and respiratory absorption from atmospheric exposure, and dust particles from commercial and residential environments [32]. Exposure to BPA in utero and through lactation is also important for the developing fetus and the neonate, respectively [33][34]. However, the main route of exposure originates from water and diet, as BPA may migrate from beverage and food packaging, as well as from dental sealants [30][35]. Interestingly, skin exposure is more severe than oral intake due to the sustained presence of BPA in the organism and the elevated plasma concentrations of unconjugated toxic BPA [36]. However, this kind of exposure does not reflect a natural setting. Moreover, Christensen et al. reported that the majority of BPA exposure was food-related (i.e., via leaching from food packaging materials and containers) after a fasting study to exclude food intake as a potential route of BPA exposure [37].
BPA and its conjugates have been detected in various body fluids and tissues, including urine, saliva, plasma, feces, amniotic fluid and breast milk; however, urine samples are mainly examined for human monitoring. Epidemiologic studies have confirmed the widespread exposure to BPA with 95 to 99.8% of adults, adolescents, children and infants presenting detectable concentrations of BPA and its metabolites in urine, independently of gender, income, educational level or BMI [38][39]. Additionally, circulating free unconjugated BPA, which is the active form of BPA, is usually determined at concentrations of nanograms per millimeter in serum or plasma [40]. BPA kinetics analyses have shown that the rate of excretion of BPA is not highly influenced by fasting, suggesting a slower rate of BPA excretion or a potential bioaccumulation of BPA in human tissues, particularly in adipose tissue [29][33]. Interestingly, in a study of the urinary BPA profile in five individuals over a 48-h period of fasting (bottled water only), BPA levels increased after the pre-fast meal, decreased over the next 24 h, fluctuated at lower levels during the second day, and then rose after the post-fast meal [37]. This rise may be attributed to non-food sources that could be still present, such as dust, or the release of BPA from lipid reservoirs from past exposures [37].
Exposure to BPA has been associated with a plethora of disorders such as obesity, T2DM, cardiovascular disease, infertility, neurodegenerative diseases and cancer, particularly breast cancer [41]. Moreover, BPA is classified as a potentially toxic and harmful substance to reproduction and eyes, respectively, and a possibly irritating substance to the skin and respiratory tract [42].
Due to increasing concerns about the safety of BPA and evidence of its relationship with human health, BPA has been banned in the manufacture of baby bottles since 2011 in the European Union (EU). The U.S. Food and Drug Administration (FDA) banned the use of BPA in baby bottles, spill-proof cups and infant formula packaging materials in 2012–2013. In April 2023, the European Food Safety Authority (EFSA) published a re-evaluation of BPA’s safety, drastically decreasing the tolerable daily intake (TDI) for BPA from 4 μg/kg of body weight per day in 2015 to 0.2 ng/kg (around 20,000 times lower than before) [43]. These restrictions were mainly based on toxicological animal studies that appraised BPA side effects on renal, hepatic and immune function [30][44]. Nevertheless, since there is an absence of international standardization regarding a tolerable BPA limit, BPA is actually used in the production of polycarbonate plastic food materials. Following the bans and restrictions, the industry has gradually employed a variety of less-studied BPA analogs with toxicological characteristics that are not fully elucidated. However, like BPA, alternatives such as bisphenols S (BPS), F (BPF), B (BPB), C (BPC), E (BPE), AF (BPAF), P (BPP) and Z (BPZ) and 4-cumylphenol (HPP) have also been identified as EDCs, presenting androgenic, estrogenic and obesogenic activity in vitro as well as neurotoxic, genotoxic and cytotoxic potential [30][41][44][45][46][47][48]. BPA analogs may bind to nuclear receptors due to their phenyl moiety and hydrophobic structure that play a role in endocrine-disrupting activity [49].
3. Phthalates
Phthalates are a group of chemical compounds in the family of esters derived from phthalic acid. The common chemical structure of phthalates involves a benzene ring with two carboxylic acid groups (ortho-phthalates, Figure 3) or one carboxylic acid group (para-phthalates) attached. The length and structure of the alkyl side chains attached to these carboxylic acid groups vary, resulting in different types of phthalates with specific properties [50]. Ortho-phthalates represent the most common type of phthalates and include diethyl phthalate (DEP), dibutyl phthalate (DBP), diisobutyl phthalate (DIBP), diisononyl phthalate (DINP) and di(2-ethylhexyl) phthalate (DEHP). Phthalates first appeared in the 1920s, and their widespread use as plasticizers destined primarily to soften polyvinyl chloride (PVC) started in the 1930s. Currently, the total production of phthalates is approximately 5.5 million tons per year with an increasing rise attributed to the use of PVC [51]. Phthalates constitute a large fraction of polymer additives due to the presence of various beneficial properties, such as flexibility, compatibility with polymers, chemical stability and durability, low water solubility, resistance to heat and weather conditions, electrical resistivity and transparency. They are primarily used as plasticizers in the production of flexible PVC products (vinyl flooring, PVC cables and wires, etc.); personal care products in cosmetics, perfumes and lotions to enhance fragrance and texture; toys and children’s products; adhesives and sealants; medical devices; building materials; coatings and inks; textiles and carpets; and food packaging [51][52].
Because phthalates are not covalently bound to their compounds, they may easily migrate and leak into the environment, where they can accumulate in the food chain due to their lipophilic nature [53]. Hence, humans are exposed to phthalates through dietary exposure, which represents the major route for intake; skin absorption; and inhalation of indoor and outdoor air comprising dust [14][54]. Infants, toddlers and children have a higher exposure to phthalate esters than adults through their mouthing activity and contact with toys, carpets and floors [14]. Furthermore, certain phthalates have the ability to pass through the placenta, potentially exposing the developing fetus to these chemicals during pregnancy [55][56]. Epidemiological studies have shown that phthalates, particularly the commonly used DEHP and its breakdown products, may be found in a plethora of human body fluids such as urine, which is the most commonly studied and used matrix for assessing human exposure to phthalates; plasma; saliva; follicular fluid; amniotic fluid; and breast milk [56][57][58][59]. Phthalate esters present endocrine-disrupting properties associated with detrimental reproductive and neurodevelopmental effects as well as obesity and T2DM [14][60][61][62]. In response to concerns about the potential harmful effects of phthalates, the use of certain phthalates including DEHP has been restricted in concentrations exceeding 0.1% by weight of the plasticized products in Europe and elsewhere [63]; nevertheless, there are no international acceptable limits for phthalate esters. Efforts have been made to reduce or replace their use in various applications with non-phthalate and bio-based plasticizers, polymer blends and formulations, and alternative materials. Interestingly, exposure to most EDCs, including DEHP metabolites and BPA, decreased between 2009 and 2016 in a sample of individuals with impaired fasting glucose from the Dutch population [64]. However, there is a need to assess the use of less toxic substitute chemical compounds for their metabolic and endocrine consequences.
This entry is adapted from the peer-reviewed paper 10.3390/ijms25010675
References
- World Obesity Federation. World Obesity Atlas 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 14 November 2023).
- Wang, Y.; Beydoun, M.A.; Min, J.; Xue, H.; Kaminsky, L.A.; Cheskin, L.J. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int. J. Epidemiol. 2020, 49, 810–823.
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the Obesity Epidemic: After the Sudden Rise, Is the Upward Trajectory Beginning to Flatten? Curr. Obes. Rep. 2023, 12, 514–527.
- NCD-RisC. Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: A pooled analysis of 2181 population-based studies with 65 million participants. Lancet 2020, 396, 1511–1524.
- Papadavid, E.; Vlami, K.; Dalamaga, M.; Giatrakou, S.; Theodoropoulos, K.; Gyftopoulos, S.; Stavrianeas, N.; Papiris, S.; Rigopoulos, D. Sleep apnea as a comorbidity in obese psoriasis patients: A cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? J. Eur. Acad. Dermatol. Venereol. 2013, 27, 820–826.
- Papavasileiou, G.; Tsilingiris, D.; Spyrou, N.; Vallianou, N.G.; Karampela, I.; Magkos, F.; Dalamaga, M. Obesity and main urologic cancers: Current systematic evidence, novel biological mechanisms, perspectives and challenges. Semin. Cancer Biol. 2023, 91, 70–98.
- Hroussalas, G.; Kassi, E.; Dalamaga, M.; Delimaris, I.; Zachari, A.; Dionyssiou-Asteriou, A. Leptin, soluble leptin receptor, adiponectin and resistin in relation to OGTT in overweight/obese postmenopausal women. Maturitas 2008, 59, 339–349.
- Pavlidou, A.; Dalamaga, M.; Kroupis, C.; Konstantoudakis, G.; Belimezi, M.; Athanasas, G.; Dimas, K. Survivin isoforms and clinicopathological characteristics in colorectal adenocarcinomas using real-time qPCR. World J. Gastroenterol. 2011, 17, 1614–1621.
- Hassan, S.; Thacharodi, A.; Priya, A.; Meenatchi, R.; Hegde, T.A.; Thangamani, R.; Nguyen, H.T.; Pugazhendhi, A. Endocrine disruptors: Unravelling the link between chemical exposure and Women’s reproductive health. Environ. Res. 2023, 241, 117385.
- Emfietzoglou, R.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Could the endocrine disruptor bisphenol-A be implicated in the pathogenesis of oral and oropharyngeal cancer? Metabolic considerations and future directions. Metabolism 2019, 91, 61–69.
- Heindel, J.J.; Alvarez, J.A.; Atlas, E.; Cave, M.C.; Chatzi, V.L.; Collier, D.; Corkey, B.; Fischer, D.; Goran, M.I.; Howard, S.; et al. Obesogens and Obesity: State-of-the-Science and Future Directions Summary from a Healthy Environment and Endocrine Disruptors Strategies Workshop. Am. J. Clin. Nutr. 2023, 118, 329–337.
- Kiess, W.; Haeusler, G. Endocrine-disrupting chemicals. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101566.
- WHO. Global Assessment on the State of the Science of Endocrine Disruptors. Available online: https://www.who.int/publications/i/item/WHO-PSC-EDC-02.2 (accessed on 28 November 2023).
- Nidens, N.; Vogel, M.; Körner, A.; Kiess, W. Prenatal exposure to phthalate esters and its impact on child development. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101478.
- National Institute of Environmental Health Sciences. Endocrine Disruptors. Available online: https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm (accessed on 28 November 2023).
- van der Meer, T.P.; van Faassen, M.; van Beek, A.P.; Snieder, H.; Kema, I.P.; Wolffenbuttel, B.H.R.; van Vliet-Ostaptchouk, J.V. Exposure to Endocrine Disrupting Chemicals in the Dutch general population is associated with adiposity-related traits. Sci. Rep. 2020, 10, 9311.
- Frederiksen, H.; Jensen, T.K.; Jørgensen, N.; Kyhl, H.B.; Husby, S.; Skakkebæk, N.E.; Main, K.M.; Juul, A.; Andersson, A.M. Human urinary excretion of non-persistent environmental chemicals: An overview of Danish data collected between 2006 and 2012. Reproduction 2014, 147, 555–565.
- Kiess, W.; Häussler, G.; Vogel, M. Endocrine-disrupting chemicals and child health. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101516.
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27.
- Plastics—The Facts 2022. Available online: https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf (accessed on 28 November 2023).
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781.
- Michałowicz, J. Bisphenol A--sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758.
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95.
- Kim, M.J.; Park, Y.J. Bisphenols and Thyroid Hormone. Endocrinol. Metab. 2019, 34, 340–348.
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34.
- Dueñas-Mas, M.J.; Ballesteros-Gómez, A.; Rubio, S. Supramolecular solvent-based microextraction of emerging bisphenol A replacements (colour developers) in indoor dust from public environments. Chemosphere 2019, 222, 22–28.
- Darbre, P.D. Chemical components of plastics as endocrine disruptors: Overview and commentary. Birth Defects Res. 2020, 112, 1300–1307.
- Seachrist, D.D.; Bonk, K.W.; Ho, S.M.; Prins, G.S.; Soto, A.M.; Keri, R.A. A review of the carcinogenic potential of bisphenol A. Reprod. Toxicol. 2016, 59, 167–182.
- Robinson, L.; Miller, R. The Impact of Bisphenol A and Phthalates on Allergy, Asthma, and Immune Function: A Review of Latest Findings. Curr. Environ. Health Rep. 2015, 2, 379–387.
- Biemann, R.; Blüher, M.; Isermann, B. Exposure to endocrine-disrupting compounds such as phthalates and bisphenol A is associated with an increased risk for obesity. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101546.
- Hoekstra, E.J.; Simoneau, C. Release of bisphenol A from polycarbonate: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 386–402.
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. Ann. Endocrinol. 2013, 74, 211–220.
- Xu, J.; Huang, G.; Guo, T.L. Developmental Bisphenol A Exposure Modulates Immune-Related Diseases. Toxics 2016, 4, 23.
- Di Credico, A.; Gaggi, G.; Bucci, I.; Ghinassi, B.; Di Baldassarre, A. The Effects of Combined Exposure to Bisphenols and Perfluoroalkyls on Human Perinatal Stem Cells and the Potential Implications for Health Outcomes. Int. J. Mol. Sci. 2023, 24, 15018.
- Hassan, R.; Aslam Khan, M.U.; Abdullah, A.M.; Abd Razak, S.I. A Review on Current Trends of Polymers in Orthodontics: BPA-Free and Smart Materials. Polymers 2021, 13, 1409.
- Liu, J.; Martin, J.W. Prolonged Exposure to Bisphenol A from Single Dermal Contact Events. Environ. Sci. Technol. 2017, 51, 9940–9949.
- Christensen, K.L.; Lorber, M.; Koslitz, S.; Brüning, T.; Koch, H.M. The contribution of diet to total bisphenol A body burden in humans: Results of a 48 h fasting study. Environ. Int. 2012, 50, 7–14.
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070.
- Olsén, L.; Lampa, E.; Birkholz, D.A.; Lind, L.; Lind, P.M. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Ecotoxicol. Environ. Saf. 2012, 75, 242–248.
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484.
- Catenza, C.J.; Farooq, A.; Shubear, N.S.; Donkor, K.K. A targeted review on fate, occurrence, risk and health implications of bisphenol analogues. Chemosphere 2021, 268, 129273.
- European Chemicals Agency, 2022. The use of bisphenol A and its alternatives in thermal paper in the EU during 2014–2022. Echa. Available online: https://echa.europa.eu/documents/10162/2564887/bpa_thermal_paper_report_2020_en.pdf/59eca269-c788-7942-5c17-3bd822d9cba0 (accessed on 2 December 2023).
- Bisphenol, A. Available online: https://www.efsa.europa.eu/en/topics/topic/bisphenol (accessed on 28 November 2023).
- Kodila, A.; Franko, N.; Sollner Dolenc, M. A review on immunomodulatory effects of BPA analogues. Arch. Toxicol. 2023, 97, 1831–1846.
- Martínez, M.; Blanco, J.; Rovira, J.; Kumar, V.; Domingo, J.L.; Schuhmacher, M. Bisphenol A analogues (BPS and BPF) present a greater obesogenic capacity in 3T3-L1 cell line. Food Chem. Toxicol. 2020, 140, 111298.
- Ramskov Tetzlaff, C.N.; Svingen, T.; Vinggaard, A.M.; Rosenmai, A.K.; Taxvig, C. Bisphenols B, E, F, and S and 4-cumylphenol induce lipid accumulation in mouse adipocytes similarly to bisphenol A. Environ. Toxicol. 2020, 35, 543–552.
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453.
- McDonough, C.M.; Xu, H.S.; Guo, T.L. Toxicity of bisphenol analogues on the reproductive, nervous, and immune systems, and their relationships to gut microbiome and metabolism: Insights from a multi-species comparison. Crit. Rev. Toxicol. 2021, 51, 283–300.
- Usman, A.; Ahmad, M. Computational study suggesting reconsideration of BPA analogues based on their endocrine disrupting potential estimated by binding affinities to nuclear receptors. Ecotoxicol. Environ. Saf. 2019, 171, 154–161.
- U.S. Environmental Protection Agency. Phthalates. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/phthalates (accessed on 1 December 2023).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782.
- Barnes, S.J. Understanding plastics pollution: The role of economic development and technological research. Environ. Pollut. 2019, 249, 812–821.
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbühler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26, 803–824.
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health 2014, 13, 43.
- Vogel, N.; Lange, R.; Schmidt, P.; Rodriguez Martin, L.; Remy, S.; Springer, A.; Puklová, V.; Černá, M.; Rudnai, P.; Középesy, S.; et al. Exposure to Phthalates in European Children, Adolescents and Adults since 2005: A Harmonized Approach Based on Existing HBM Data in the HBM4EU Initiative. Toxics 2023, 11, 241.
- Xia, B.; Zhu, Q.; Zhao, Y.; Ge, W.; Zhao, Y.; Song, Q.; Zhou, Y.; Shi, H.; Zhang, Y. Phthalate exposure and childhood overweight and obesity: Urinary metabolomic evidence. Environ. Int. 2018, 121, 159–168.
- Govarts, E.; Gilles, L.; Rodriguez Martin, L.; Santonen, T.; Apel, P.; Alvito, P.; Anastasi, E.; Andersen, H.R.; Andersson, A.M.; Andryskova, L.; et al. Harmonized human biomonitoring in European children, teenagers and adults: EU-wide exposure data of 11 chemical substance groups from the HBM4EU Aligned Studies (2014–2021). Int. J. Hyg. Environ. Health 2023, 249, 114119.
- Li, Y.; Xiao, N.; Liu, M.; Liu, Y.; He, A.; Wang, L.; Luo, H.; Yao, Y.; Sun, H. Dysregulation of steroid metabolome in follicular fluid links phthalate exposure to diminished ovarian reserve of childbearing-age women. Environ. Pollut. 2023, 330, 121730.
- Liu, Y.; Xiao, M.; Huang, K.; Cui, J.; Liu, H.; Yu, Y.; Ma, S.; Liu, X.; Lin, M. Phthalate metabolites in breast milk from mothers in Southern China: Occurrence, temporal trends, daily intake, and risk assessment. J. Hazard. Mater. 2023, 464, 132895.
- Ferguson, K.K.; Rosen, E.M.; Rosario, Z.; Feric, Z.; Calafat, A.M.; McElrath, T.F.; Vélez Vega, C.; Cordero, J.F.; Alshawabkeh, A.; Meeker, J.D. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ. Int. 2019, 132, 105099.
- Choi, G.; Villanger, G.D.; Drover, S.S.M.; Sakhi, A.K.; Thomsen, C.; Nethery, R.C.; Zeiner, P.; Knudsen, G.P.; Reichborn-Kjennerud, T.; Øvergaard, K.R.; et al. Prenatal phthalate exposures and executive function in preschool children. Environ. Int. 2021, 149, 106403.
- Stahlhut, R.W.; van Wijngaarden, E.; Dye, T.D.; Cook, S.; Swan, S.H. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ. Health Perspect. 2007, 115, 876–882.
- Commission Regulation (EU) 2018/2005 of 17 December 2018 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards bis(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), benzyl butyl phthalate (BBP) and diisobutyl phthalate (DIBP) (Text with EEA relevance.). Available online: https://eur-lex.europa.eu/eli/reg/2018/2005/oj (accessed on 1 December 2023).
- van der Meer, T.P.; Chung, M.K.; van Faassen, M.; Makris, K.C.; van Beek, A.P.; Kema, I.P.; Wolffenbuttel, B.H.R.; van Vliet-Ostaptchouk, J.V.; Patel, C.J. Temporal exposure and consistency of endocrine disrupting chemicals in a longitudinal study of individuals with impaired fasting glucose. Environ. Res. 2021, 197, 110901.
This entry is offline, you can click here to edit this entry!
