Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Genetics & Heredity
Major depressive disorder (MDD) is a complex disorder and a leading cause of disability in 280 million people worldwide. Many environmental factors, such as microbes, drugs, and diet, are involved in the pathogenesis of depressive disorders.
- major depressive disorder
- gut microbiome
- diet
- probiotics
1. Introduction
Depressive disorders are among the most common and complex emotional mental disorders, which annually influence 350 million people worldwide with an average lifetime prevalence of 11–15% [1,2]. Depressive disorders occur across wide ranges of ages from childhood to late life and inflict a high cost on society [2,3]. The prevalence of depressive disorders has doubled or even higher during the COVID-19 pandemic [4]. Depressive disorders are generally characterized by the loss of interest, depressed mood, hopelessness, feelings of guilt or worthlessness, lack of energy, poor concentration, appetite changes, psychomotor retardation or agitation, anxiety, and sleep disturbances causing problems in daily routines, and sometimes are associated with suicidal ideas [5]. When a depressive disorder lasts for a prolonged period of time with moderate or severe intensity, it causes a serious health condition, which is called major depressive disorder (MDD). Recurrence of depressive episodes with stronger severity and less responsiveness to conventional therapeutic approaches has been found among people with MDD, which in turn impacts the quality of life and increases the risk of suicide [6].
Many genetic and environmental factors, such as illicit or prescribed drugs, psychological stress, microbes, and diet, are involved in the pathogenesis of depressive disorders. However, the underlying mechanism of disease pathogenesis is complex and includes the interplay of various key factors related to genetic, epigenetic, and the host immune system, among others. Regarding molecular events that underlie depression, current knowledge greatly supports the monoamine hypothesis of depression [7]. Based on this hypothesis, any perturbation that affects brain serotonin, dopamine, or norepinephrine signaling pathways may induce depression [8,9]. Hence, most of the current antidepressants are designed to increase the level of serotonin (e.g., serotonin-specific reuptake inhibitors), dopamine (e.g., bupropion), noradrenaline (e.g., desipramine), a combination of these neurotransmitters (e.g., serotonin-noradrenaline specific reuptake inhibitors) or inhibit monoamine oxidase enzymes that degrade these neurotransmitters (e.g., monoamine oxidase inhibitors) [10,11,12]. As depicted in Figure 1, several enzymes/genes are involved in processing amino acids to produce these neurotransmitters and several others are involved in their receptors production, their transport or degradation which are targets of current therapeutics [13,14]. For example, serotonin is produced by Tph1 and 2 enzymes in the gut and brain (respectively) from tryptophan, and dopamine is produced from tyrosine (which itself is produced from phenylalanine) by TH (tyrosine hydroxylase) [15,16]. Dopamine then may be processed by DBH (Dopamine β-Hydroxylase) to produce noradrenaline. These neurotransmitters are involved in mood regulation, executive functioning, cognition, motivation, and intellectual function, fundamental aspects of social relationships [17]. MDD also arises from the disruptions of various other neurotransmitter systems, including γ-aminobutyric acid (GABA) and the glutamatergic systems [18,19]. Reduced glutamate levels in specific brain regions correlate with altered emotional responses, offering insights into MDD’s complex neurobiological underpinnings beyond classical neurotransmitters [20].
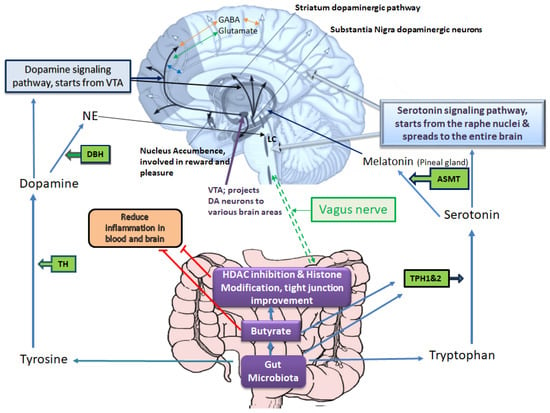
Figure 1. Gut microbiota–host interplays in tryptophan and tyrosine metabolism, which affect mood and emotional states. Based on the monoamine hypothesis of depression, disruptions in brain serotonin, dopamine, or norepinephrine pathways may induce depression. The serotonin signaling pathway starts from the raphe nucleus and spreads to the entire brain, positively influencing the nucleus accumbence responsible for reward and pleasure. The enzymes TPH1 and TPH2 are involved in serotonin synthesis, and TH is involved in dopamine synthesis. Serotonin and dopamine can further proceed to produce melatonin and noradrenaline, respectively. In brain tissue, these neurotransmitters interact with glutamate (excitatory) and GABA (inhibitory) neurotransmitters, which are also implicated in the pathogenesis of depression. The gut microbiota and its metabolites play a key role in providing substrates and influencing enzymes involved in dopamine and serotonin synthesis, consequently affecting noradrenaline (particularly in locus coeruleus, LC) and melatonin (in the pineal gland) production. Melatonin regulates circadian rhythms, which are often disrupted in depression. Additionally, gut microbiota and its metabolites, especially butyrate, help mitigate blood and brain inflammation and influence the activity of the vagus nerve, which directly communicates with the brain in the medulla oblongata. The red T-shape merks indicate inhibition.
Among environmental factors, the gut microbiota composition is a key player in serotonin and dopamine production, which may play significant roles in delaying or accelerating depression by regulation of tryptophan availability and, subsequently, serotonin synthesis in the gut and brain cells [21,22]. The gut microbiome–derived metabolites such as butyrate are capable of enhancing the expression and Tph1 activity [23,24,25]. The production of serotonin in the gut, in turn, can influence brain functions via stimulation of the vagus nerve [21], while blood serotonin level affects the blood-brain-barrier (BBB) permeability as well [21]. Moreover, butyrate and epigenetic drugs with similar effects (e.g., sodium valproate) can promote the production of dopamine, noradrenaline, and other related neurotransmitters via enhancing the transcription of the tyrosine hydroxylase (TH) gene [26,27].
Other research findings also indicate that modifications of the gut microbiome and its metabolites influence stress-related responses and social behavior in patients with depressive disorders by modulating the maturation of the brain’s immune cells and neurogenesis. In fact, the gut microbiota and its metabolites are involved in a large number of physiological processes like nutrient absorption, strengthening of the intestinal epithelial barrier, facilitating maturation of immune cells, improving functionality of the host immune system, and regulating brain function and human behavior [28,29,30,31]. While the number of microbes in the human gut is several-fold higher than the 30–40 trillion human body cell number, the collective genes of more than 1000 different types of gut microbes are 100–150-fold greater than the human 30,000 genes [32,33]. After the conception of the idea of the microbial–gut–brain axis, it is increasingly becoming clear that this axis is a dynamic, complex, bidirectional communication path that mediates the connection between the gastrointestinal tract (GIT) and the central nervous system (CNS) via several ways including the vagus nerve, neurotransmitters, hormones, microbial metabolites, and the immune system. This axis displays a powerful role in numerous physiological processes like the brain microglia function, the blood–brain barrier integrity and its permeability, and the activity of peripheral immune system cells [34,35]. Accumulating evidence has shown that gut microbiota can be considered as an environment-linked factor that not only shapes the brain via the production of different neurotransmitters but also affects the production of short-chain fatty acids (SCFAs) such as butyrate, acetate, and propionate, which are strong epigenetic modifiers of many genes contributing to the microbiota-gut-brain axis functions.
Various environmental factors like diet and dietary habits, psychosocial stress, sedentary lifestyle, smoking, antibiotic consumption, chemical substances, and pesticides influence the composition of gut microbiota and the development of depressive disorders [36]. For example, mice with depressive disorders-related behaviors often exhibit altered gut microbiome [37,38]. Furthermore, a growing body of evidence has shown that gut microbiota alterations are associated with the dysregulation of epigenetic mechanisms (e.g., DNA methylation, histone modification, and non-coding RNA-associated gene silencing) and, thereby, the development of depressive disorders [39,40]. Non-coding RNA-associated gene silencing, which is mainly mediated by microRNAs (miRNAs), works between the gut microbiome and intrinsic host factors. For instance, there are significant differences in the expression of miR-294-5p (a miRNA responsible for targeting some key genes in the kynurenine pathway) between germ-free (GF) male mice and conventional mice [41]. Another study by Stilling et al. reported reduced expression of miR-183-5p and miR-182-5p in the amygdala region of the GF mice, and its restoration following microbiome recolonization [42].
2. Roles of Leaky Gut in the Pathophysiology of Depressive Disorders via Alterations of Gut Microbiota-Derived Metabolites Affecting the Epigenetic Landscape
The intestinal epithelial barrier is composed of a single cell layer that is responsible for the delimitation of the internal milieu from the luminal environment. A major advantage of this restriction is the prevention of entering an expensive range of factors (toxins, pathogens, and antigens) into the lumen, which further results in inflammation, infection, and changes in normal body function [43]. Paracellular transportation across the intestinal epithelium is regulated by tight junctions between adjacent intestinal epithelial cells [44]. The integrity of tight junctions is regulated by endogenous (neural and humoral signals and inflammatory mediators) and exogenous (diet and bacterial metabolites) factors [45]. Some other environmental factors, such as stressful life events, may enhance intestinal permeability and increase the risk of various gastrointestinal disorders and, subsequently, the onset and development of depressive disorders [46,47].
It is well-known that gut microbiota is also capable of regulating motility barrier function and visceral perception, and its diversity and composition can by altered by environmental factors, which in turn impact the integrity of tight junctions and hence accelerate (or delay) the development of depressive disorders [48,49,50]. It has been found that an enhanced gastrointestinal permeability with an increased LPS (lipopolysaccharide) translocation from gram-negative bacteria into the blood circulation displays a potent role in the pathophysiology of depressive disorders via activation of the inflammatory response system [46]. In addition to activation of the inflammatory responses, bacterial translocation plays an important role in driving oxidative and nitrosative stress in patients with depressive disorders [51]. The susceptibility of the intestinal barrier is increased secondary to opportunistic microbial inhabitants in a state of microbial dysbiosis. This, in turn, results in the deterioration of the epithelium functions, enhancing localized inflammation, generating inflammatory microbial byproducts, perturbing the functions of other tissues, including the brain, via blood circulation, and increasing the risk of mood-related [52]. Contaminant residues in food products like pesticides are also considered environmental factors with the capacity to disturb gut permeability and enhance inflammation by altering gut microbiome composition [53]. Moreover, it has been reported that psychological stress, such as chronic exposure to limited nesting stress during the first week of the post-natal period, results in enhanced intestinal permeability, reducing fecal microbial diversity, decreasing the abundance of fiber-degrading, butyrate-producing, and mucus-resident microbes as well as increasing the abundance of Gram-positive cocci [54]. The gut microbiota is a key contributor to such effects via their capacity to either enhance or protect against inflammation involving factors affecting epigenetic regulations. For instance, the depletion of bacteria, which produce anti-inflammatory and barrier-strengthening molecules such as butyrate (that enhances the expression of tight junction proteins), results in gut barrier disruption and a loss of protection against epithelial inflammation. Firmicutes bacteria of the gut microbiota are also capable of fermentation of carbohydrates to different SCFAs such as butyrate, acetate, and propionate, while the lack of SCFAs (as important and widespread epigenetic modifiers) gives rise to the disruption of intestinal barrier function and secondary inflammation [55].
The maintenance of microbial health is largely dependent on the enrichment of microbial SCFA producers and microbial diversity, which are associated with optimal immune function and intestinal barrier integrity. Among SCFAs, butyrate is a master regulator of biological responses of host gastrointestinal health via inhibition of histone deacetylases (HDACs) and binding to specific G protein-coupled receptors (GPCRs) [56]. Butyrate displays a crucial role in numerous physiological processes by crossing across the BBB, activation of the vagus nerve and hypothalamus, and promoting the cholinergic neurons through epigenetic mechanisms [57,58]. Elevated butyrate in the gut contributes to promoting epithelial barrier integrity and inhibiting the host’s systemic inflammation via activation of regulatory T cells [59]. In addition to the activation of regulatory T cells, this gut microbiota metabolite enhances the expression of lncLy6C, which further results in promoting the differentiation of Ly6Chigh pro-inflammatory monocytes into Ly6Clow/neg resident macrophages. This is via binding to the transcription factor C/EBPβ and multiple lysine methyltransferases of H3K4me3 (tri methylation of lysine 4 of the DNA packaging protein Histone H3) and hence improving the enrichment of C/EBPβ and H3K4me3 marks on the promoter region of Nr4A1 gene involved in inflammation regulation and neuroprotection [60]. Moreover, in the CNS, butyrate can enhance the expression of brain-derived neurotrophic factor (BDNF), a main mediator of antidepressant-like effects in animal models, via influencing the hippocampus function [61]. Diet and other environmental factors are considered master regulators of butyrate-producing bacteria and, thereby, the maintenance of intestinal barrier integrity. For example, an omega-3-rich diet is capable of increasing the abundance of butyrate-producing bacteria like Subdoligranulum [62].
3. The Gut Microbiome Composition and Its Role in the Development of Depressive Disorders via Epigenetic Alterations
Several animal studies uncovered that the gut microbiome composition displays a key role in the onset and development of depressive disorders, and altered microbiota profile in the colon has been reported in different animal models of depressive disorders [37,63,64]. Furthermore, significant increases in Arthromitus and Oscillibacter but decreases in the abundances of several others (e.g., Lactobacillus, Marvinbryantia, and Clostridiales incertae sedis) associated with the deterioration of intestinal barrier function and alterations in the fecal metabolites involved in tryptophan (the precursor of serotonin) metabolism have been found in stress-induced depressed rats [65]. Stress-induced behavioral changes in mice have also been linked to an increase in the abundance of Odoribacter and Alistipes bacteria correlated with higher blood IL-1α and IFN-γ levels [66]. More evidence of the relationship between depression and altered microbiota composition comes from studies that have shown fecal transplantation of stress-induced depressed mice to normal mice induces depressive phenotypes in the recipient mice due to a decrease in the production of fatty acids (known epigenetic modifiers) that can be treated with a strain of Lactobacilli [67]. In another study, while the “relative abundance of Firmicutes, Actinobacteria and Bacteroidetes” was altered in patients with MDD, fecal microbiota transplantation from these patients to germ-free mice induced depressive-like symptoms in the recipient mice but not in germ-free mice receiving fecal microbiota transplantation from normal individuals [68].
In humans, a survey of a large (>1000) cohort followed by validation in independent data sets of >1000 individuals, it was found that the abundance of two butyrate-producing bacteria (Faecalibacterium and Coprococcus) correlate with better life quality, while Dialister and, Coprococcus spp. were reduced in depression [69]. In another recent human study on >2500 individuals, depressive symptoms were associated with the abundance of a large number of bacteria (including genera Hungatella, LachnospiraceaeUCG001, Lachnoclostridium, Eubacterium ventriosum, Eggerthella, Sellimonas, Subdoligranulum, Coprococcus, Ruminococcaceae, Ruminococcusgauvreauii group, and Ruminococcaceae family) which are involved in the synthesis of butyrate, γ amino butyric acid, glutamate and serotonin, key players in depressive disorders [70]. Another clinical study in young adults showed that subjects with MDD exhibited higher levels of specific taxa like Flavonifractor and Gammaproteobacteria and lower levels of butyrate-producing, anti-inflammatory bacteria such as Subdoligranulum and Faecalibacterium [71]. In addition to changes in the abundance of bacteria that produce butyrate (which is involved in histone acetylation, an open chromatin state), alteration of diverse bacterial species may also affect DNA methylation or miRNAs. For example, in a clinical study of obese and non-obese patients with polycystic ovary syndrome (POCS) and higher depression scores vs. controls, while several gut bacteria exhibited significant alterations, this was associated with DNA hypomethylation of the FKBP5 gene, which together with NF-κB mediates inflammation and stress responses, and its increased expression is associated with impaired stress responses and unresponsiveness to antidepressants [72]. Regarding miRNA alterations, the abundances of specific genera such as Bacteroides and Dialister in MDD patients were highly correlated with the expression of several miRNAs involved in the function of MDD-associated and neurotrophins signaling pathway, circadian rhythm, and dopaminergic synapses among others [73] addressing the involvement of microbiota-miRNA interactions in the microbiota-gut-brain axis.
4. Depression and Maternal Diet and Environmental Contaminants Which Affect the Gut Microbiome and Epigenome
Animal studies have shown that maternal diet during gestation plays an essential role in the health and the neurodevelopment of offspring by modulating the gut microbiome and its metabolites [87]. Unhealthy modern diets such as high-fat diets are capable of inducing maternal dysbiosis, reducing the abundance of butyrate-producing bacteria like Firmicutes phylum, associated with an increase in anxiety-/depressive-like behaviors in male and female offspring in mice [88]. Moreover, prolonged high-fat diet feeding could reduce maternal gut SCFAs level, enhancing inflammation, decreasing the abundance of neuroactive metabolites in maternal milk during nursing, and hence increasing anxiety and depressive -like behaviors in both juvenile and adult offspring of obese dams [89]. However, maternal probiotic treatment was capable of increasing gut butyrate and brain lactate in these juvenile and adult offspring, exerting a long-lasting effect on offspring neuroplasticity and their gut–liver–brain metabolome, and thereby promoting resilience to emotional dysfunction induced by maternal obesity [89]. It has been reported that maternal prebiotics (nutrients that influence gut bacterial composition) also affect fecal levels of some bacteria and brain gene expression and behavior in young and adult offspring. For example, maternal Galacto-oligosaccharide prebiotic supplementation in mice could enhance fecal butyrate and propionate levels and reduce anxiety in adult offspring [90]. On the other hand, a maternal low-fiber diet gave rise to impairment of neurocognitive functions and synaptic plasticity in offspring through altering SCFA levels, but butyrate intake could prevent these problems via epigenetic alterations [91]. High-dietary fiber intake could also reduce antenatal obesity-induced postpartum depressive disorders in the maternal mice after the offspring weaning by re-shaping the gut microbiome and increasing the formation of SCFAs (butyrate, acetate, and propionate), and hence suppressing neuroinflammation [92].
Early life exposure to environmental chemicals such as pesticides is another common pathogenesis hallmark of depressive disorders that may be associated with the disturbances of gut microbiome structure [93,94]. As an example, low-dose exposure to chlorpyrifos (a common pesticide) during early developmental periods confers perturbations of the gut-brain axis and, thereby, neurobehavioral deficits in offspring [95,96]. Some other pesticides, like glyphosate, by crossing the placental barrier and BBB, exert adverse impacts on neuroplasticity, neurodevelopment, and neuropsychiatric disorders, possibly via changing the gut microbiota and epigenetic programing [97,98,99]. Low-dose exposure to glyphosate during pregnancy and the lactational period also induces anxiety-/depressive-like behaviors and social behavior deficits in female offspring via perturbations of the gut-brain-axis and epigenetic alterations. In an interesting recent study, Buchenauer et al. chronically exposed Balb/cByJ mice (dams) to low doses of glyphosate during pregnancy and the lactational period to examine the effects of maternal glyphosate exposure on the composition of the gut microbiota and the induction of anxiety/depressive-disorders-like behaviors in female offspring [74]. Their results revealed that glyphosate-induced DNA hypermethylation of the TPH2 (involved in serotonin synthesis in the CNS) was associated with its reduced expression in the hippocampus of female offspring and inducing depressive/anxiety-like behaviors as well as social activity deficits. Moreover, changes in the gut microbiota (reduced abundance of Akkermansia, butyrate- and propionate-producing bacteria, and elevated abundances of Alistipes and Blautia bacteria relevant to tryptophan metabolism and depressive disorders) were observed in female offspring after maternal glyphosate exposure. Figure 1. illustrates the connection between gut microbiota products such as butyrate and tryptophan which affect gut and brain serotonin level which in turn is processed to produce melatonin (a key player in circadian rhythm regulation) along with its other functions in mood regulation.
This entry is adapted from the peer-reviewed paper 10.3390/genes14122217
This entry is offline, you can click here to edit this entry!
