The most common kind of cancer among women is acknowledged to be breast cancer, which has a considerable influence on both longevity and quality of life. Traditional treatments, namely, surgery, radiation, and chemotherapy, help to manage the cancer, but do not provide a long-term cure [
21]. Numerous signals coming from the environment around cancer cells have a significant impact on how the tumor develops. It has been proposed that bone marrow may operate as a storage location for dormant tumor cells that recirculate and infect other organs when conditions are suitable [
22]. However, studies have shown both tumor-promoting and tumor-suppressive responses, making the impact of MSCs on tumor growth inconsistent [
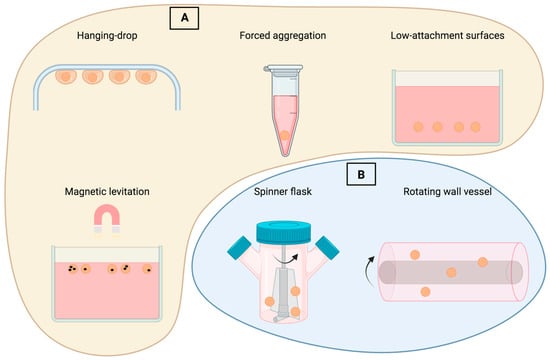
23]. Collectively, it is clear that understanding the mechanisms behind MSC-mediated modification of tumor cell activity is essential for both developing novel, precise therapies and ensuring the safe application of MSCs in the clinic. To better understand MSC biology and improve MSC-based therapeutics, there has been a rising interest in employing 3D nonadherent culture platforms, which are often employed as tumor models and in drug development [
24,
25,
26,
27]. A unique platform for high-throughput analysis of molecular alterations during the start, development, and metastasis of breast cancer may be provided by the use of 3D cell culture models, which have improved our understanding of how breast cancer progresses and allowed us to test new therapies. However, new models are required which yield reproducible quantitative data that better mimic the mammary gland architecture.
2.1. MSCs as a Tool towards Novel Anti-Cancer Therapeutic Targets
Non-adherent cultures have been utilized successfully to explore certain cell engulfment programs, often known as cell cannibalism, according to a paper by Bartosh et al. Generally speaking, cell cannibalism—also known as xenocannibalism—describes a process in which a cell encloses and ultimately destroys one or more target cells that are either nearby and of the same (homotypic) kind or of a distinct (heterotypic) kind [
34]. Cell cannibalism, a live-cell feeding activity, is regarded to be different from the traditional phagocytosis that macrophages utilize to destroy apoptotic cells from a molecular standpoint [
35]. Although one result of entosis, akin to cell cannibalism, is the death of the internalized cell, it is also thought to be separate from the live-cell engulfment processes of entosis and emperipolesis, which require active invasion/penetration of one cell into the cytoplasm of another [
36,
37]. The researchers focused on the interactions between bone marrow-derived MSCs and MDA-MB-231 (MDA) breast cancer cells (BCCs) in this investigation using hanging drop cultures. The results showed that BCCs under stress could consume or “cannibalize” MSCs in these 3D cocultures, a process that mirrored, from a morphological perspective, the rare but well-documented clinical phenomenon of cancer cell cannibalism and that boosted inflammatory response and cell sustainability while stalling tumor formation.
Because there was no evidence of cell cannibalism in 2D adherent cultures or in vivo following coinjections of MDA cells and MSCs, the 3D tumor niche model was crucial for enhancing cell feeding behaviors and determining the effects of cannibalism. Microarray experiments were used to further evaluate the MDA phenotype, and it was shown that cannibalism of MSCs caused an up-regulation of several cytokines and chemokines. Inflammatory mediators like IL-1, IL-1, IL-6, IL-8, CXCL1, CXCL2, GCSF, and PAI-1 (SERPINE1), all of which were up-regulated after MSC cannibalism, are byproducts of senescent cells and are important components of the senescence-associated secretory phenotype [
38,
39].
The development, growth, and invasiveness of cancer depend heavily on interactions between tumor cells and their microenvironment. Mesenchymal stem cells in particular are attracted to areas of growing malignancies, encouraging the development of metastases. Although the migration and integration of MSCs in the tumor microenvironment (TME) are well documented, the role of MSCs is still unclear after they reach the tumor. According to data, breast cancer cells that interact with MSCs proliferate and metabolically function more actively, in part because of MSC-derived microvesicles that are released into the TME [
40,
41].
It is unclear exactly how MSCs cause cancer cells to invade. Using a 3D coculture model, McAndrews and his team examined how MSCs influence the migration of invasive breast cancer cells. Breast cancer cells’ elongation, directional migration, and traction creation are all increased in coculture with MSCs. Transforming growth factor (TGF-β) and the migratory proteins Rho-associated kinase, focal adhesion kinase, matrix metalloproteinases, and MSC-induced directional migration all directly connect with traction production.
It was previously demonstrated that MCF7, the cell type employed by McAndrews et al., was mainly non-motile in collagen gels, most likely because it had an epithelial character [
44]. Epithelial-to-mesenchymal transition (EMT), which results in a more motile phenotype, can be seen in breast cancer cells treated with MSC conditioned media or TGF-β; however, this transformation takes place after 3–7 days of exposure to these stimuli [
45,
46]. Although MCF7 cells exhibit certain EMT markers, heterogenous expression of E-cadherin and vimentin was detected as well. This indicated that these cells had not yet fully made the transition to a mesenchymal phenotype. The chromatin structure of MCF7 cells does not permit complete EMT, according to other research [
47]. Together, these data show that the epithelial phenotype of MCF7 cells prevents MSCs from inducing the migration of MCF7 cells on the time scale examined in motility assays. Longer coculture trials may lead to EMT and MCF7 cell migration.
2.2. MSC Secretome as a Co-Treatment with Traditional Therapy
Doxorubicin (Dox), which has historically been used as a chemotherapy drug to treat breast cancer, has side effects that are still of major clinical concern. The use of MSCs’ secretomes has been studied concerning its active function in immunomodulation and regeneration processes, highlighting its tremendous potential in many pathological disorders [
55,
56]. Its effects in a cancer environment have not yet been identified. The most recent investigation, published by Serras et al., sought to comprehend how therapeutically relevant dosages of Dox in conjunction with the secretomes of MSCs affect both tumor and non-tumor cells. Novel methods are required in order to clarify the use of CM from 2D and 3D MSC cultures in both cancer and normal-type cells [
57]. In this regard, the study sought to further clarify the putative underlying mechanisms through a thorough mechanistic proteomic analysis, aiming to assess the effects of the secretomes of MSCs in human malignant breast cells and non-tumor cells (i.e., normal breast epithelial cells and cardiomyocytes) upon co-treatment with Dox. All in all, the findings demonstrated that tumor and non-tumor cells responded differently to the secretomes of MSCs.
2.3. Innovation of the Scaffold-Free, 3D Breast Organoid Model
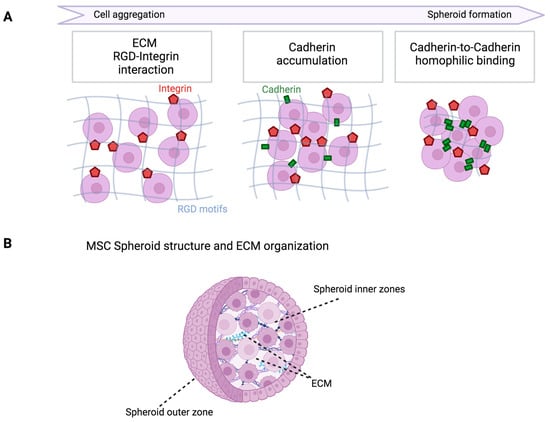
Three-dimensional cellular models are valuable for studying biological processes, while gel-embedded organoids exhibit a high degree of variability. The major benefits of the scaffold-free, 3D breast organoid model developed by Dr. Djomehri were presented in their latest study. High consistency and replication of the 3D model and the ability to measure cellular collagen I production without interference from exogenous collagen were reported with this model, as well as the ability to subject the organoid to a variety of microenvironmental and exogenous treatments at precisely timed intervals without worrying about matrix binding [
58]. By using this approach, primary metaplastic mammary carcinomas from MMTV-Cre;Ccn6fl/fl mice were converted into organoids that preserved the high-grade spindle cell shape of the original tumors. The platform is intended to be used as a standardized 3D cellular model to investigate how breast carcinogenesis is influenced by the microenvironment and to investigate new treatments. Non-tumorigenic mammary MCF10A cells, MDA-MB-231 breast cancer cells, cocultures with MCF10A and MSC cells, and primary carcinomas from MMTV-cre;Ccn6fl/fl mice were cultured using a scaffold-free 384-well hanging drop system (
Figure 2). According to the results, MCF10A cells were able to grow into organoids with cellular phenotypes that mirrored the structure of a normal human breast. In 3D suspension culture, it was discovered that laminin-rich components—rather than a laminin-rich matrix—along with a straightforward crowding agent and FBS are sufficient to support the formation of mammary acinar structures with high reproducibility. Employing scaffold-free culture MCF10A cells formed a sizable, self-organized organoid structure with the ability to display multiple lineage phenotypes [
58]. The team was able to create neoplastic organoids that had repeatable, homogeneous sizes and shapes, making it easier to standardize them against other factors. The organoids suffered cellular-level phenotypic alterations and organoid-level morphologic changes that might be measured to stratify organoid responses when subjected to settings that resemble neoplastic development. High-throughput testing and assay standardization are made possible by the simplicity of the one droplet, one organoid method, the regular form and size of the produced organoids, and the drastic morphological alterations brought on by contact with neoplastic environments. The system is intended to be used as a standardized 3D cellular model to investigate how breast carcinogenesis is influenced by the microenvironment and to investigate new treatments.